1-azabenzanthrone-platinum (II) complex as well as synthesis method and application thereof
A technology of benzanthrone and its synthesis method, which is applied in the field of medicine, can solve problems such as blank research on metal complexes, and achieve significant in vitro anti-tumor activity and good medicinal value.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Weigh the same amount of 1-azabenzanthrone and dichlorobis(dimethylsulfoxide) platinum (II), each 1mmol, dissolve 1-azabenzanthrone in 50mL of 100 % (volume) in methanol, dichlorobis(dimethylsulfoxide) platinum (II) was dissolved in 20mL of water, the two solutions were mixed, 2mL dimethylsulfoxide was added to the mixture, at 70°C The reaction was carried out at low temperature for 20 hours, concentrated and evaporated to remove most of the solvent (85% of the added amount of solvent), cooled to room temperature and allowed to stand, and the reddish-brown target product was precipitated (yield: 90%).
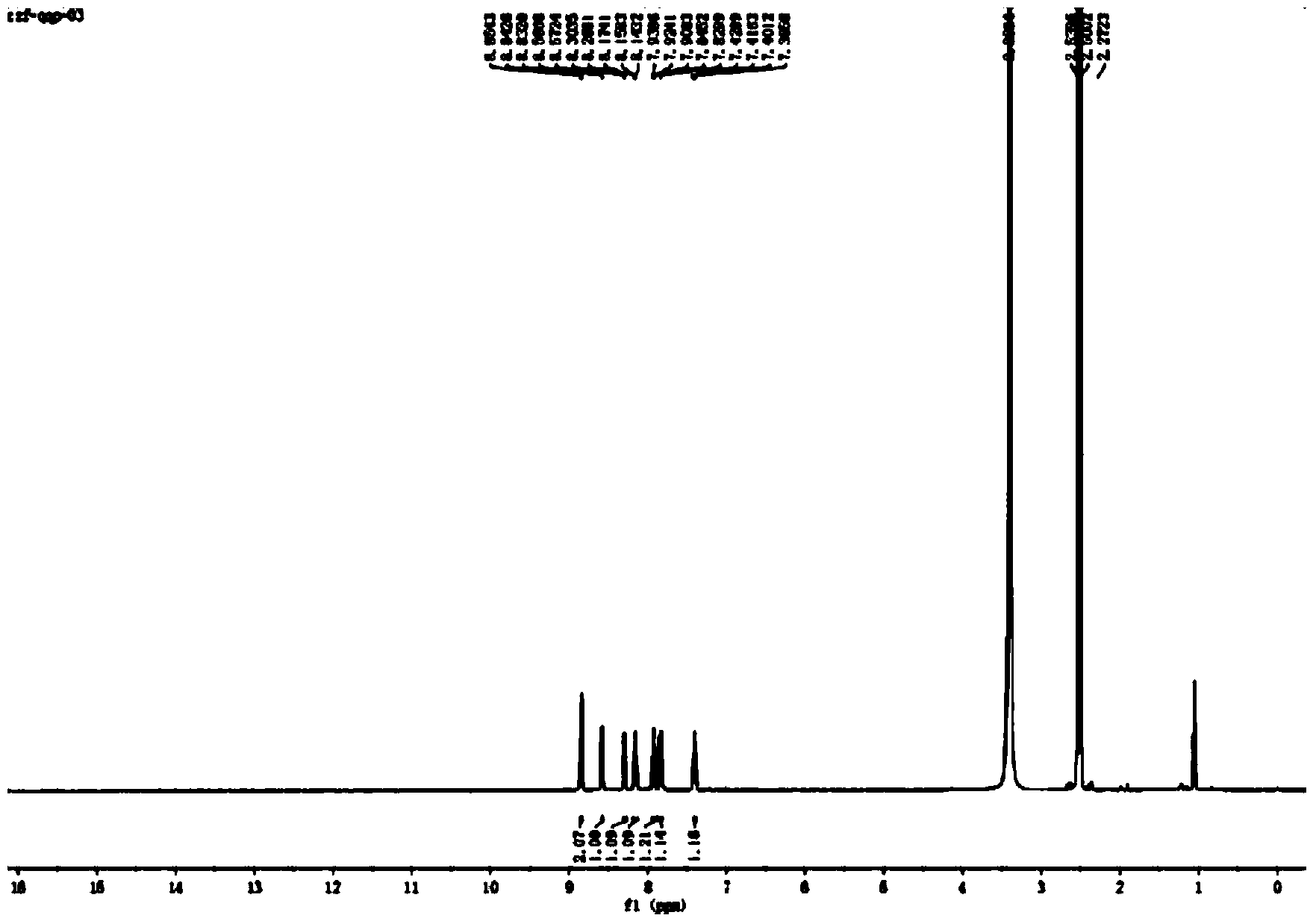
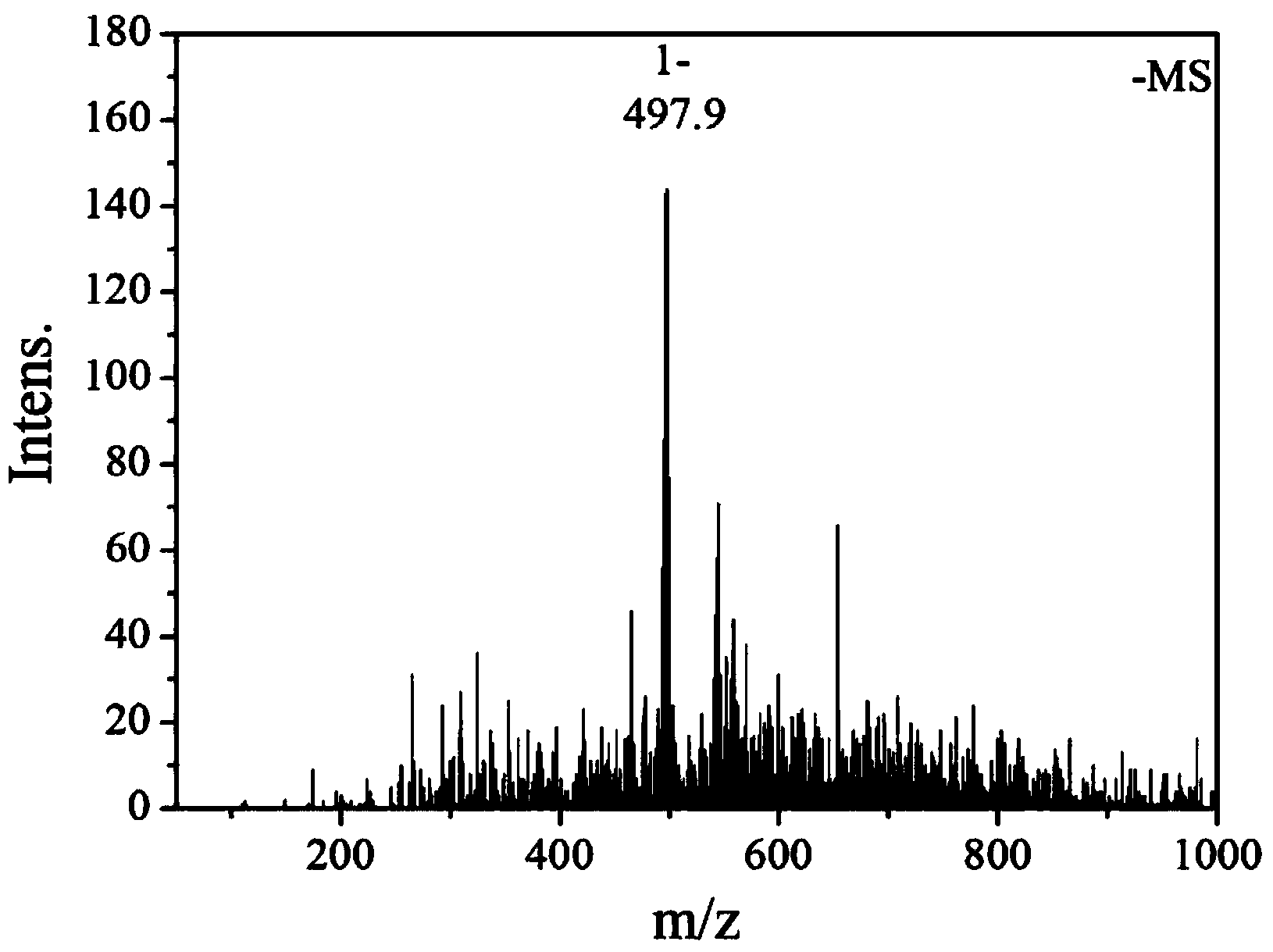
[0037] The resulting reddish-brown solid product was analyzed by infrared spectroscopy, hydrogen nuclear magnetic resonance spectroscopy, electrospray mass spectroscopy, single crystal diffraction and ultraviolet spectroscopy. The specific spectral characteristics are as follows:
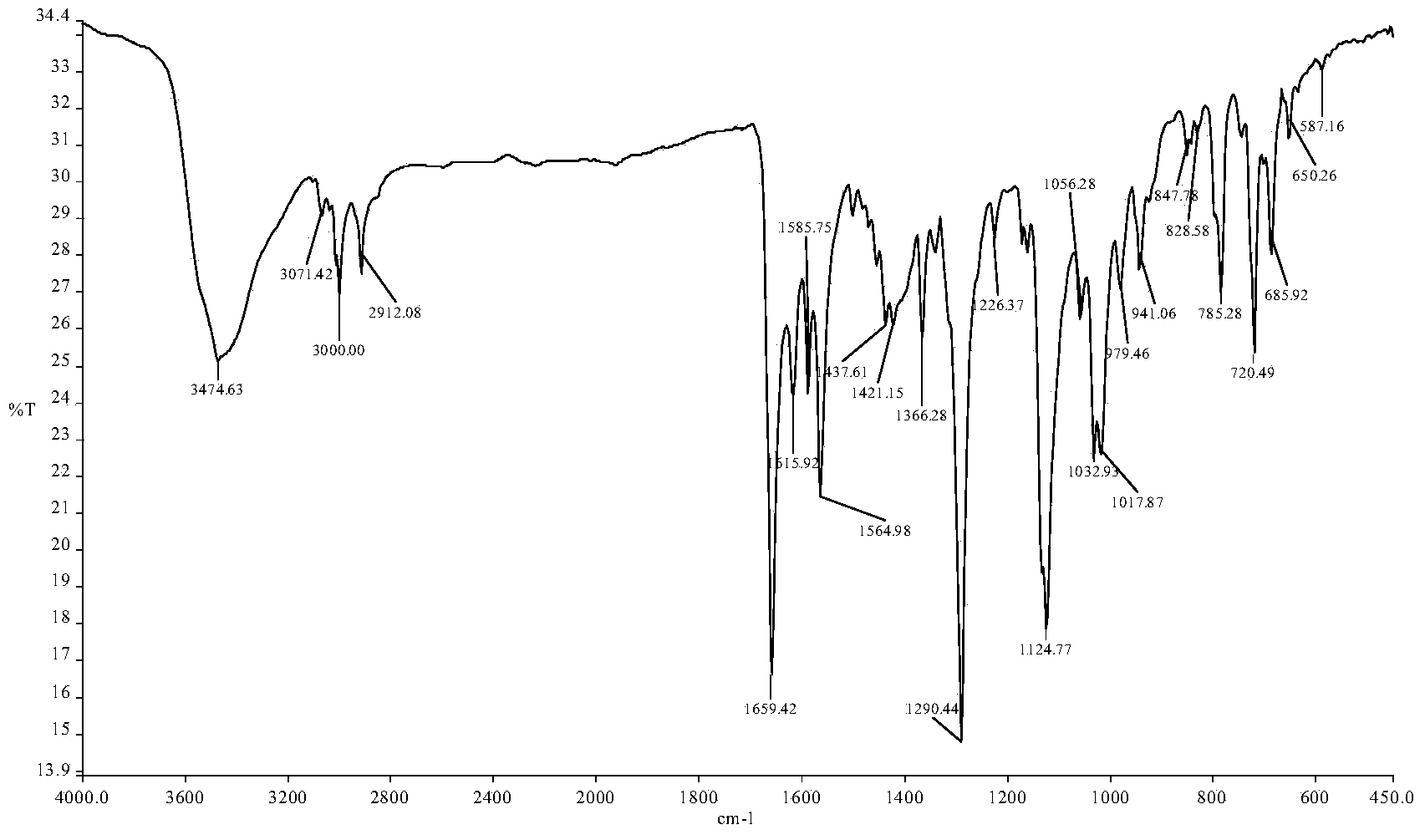
[0038] (1) Infrared spectrum, its spectrogram is as follows figure 1 as shown,
[0039] ...
Embodiment 2
[0049] Weigh the same amount of 1-azabenzanthrone and dichlorobis(dimethylsulfoxide) platinum (II), each 1mmol, dissolve 1-azabenzanthrone in 60mL of 80 % (volume) ethanol, dichlorobis(dimethylsulfoxide) platinum (II) was dissolved in 20mL of 90% (volume) ethanol, the two solutions were mixed, and 8mL dimethyl Sulfoxide, reacted at 80°C for 5 hours, concentrated and evaporated to remove most of the solvent (80% of the solvent added), cooled to room temperature and stood still, and the reddish-brown target product monochloro-mono(dimethylsulfoxide) was precipitated. Mono(1-azabenzanthrone)platinum(II) (65% yield).
Embodiment 3
[0051] Weigh the same amount of 1-azabenzanthrone and dichlorobis(dimethylsulfoxide) platinum (II), each 1mmol, dissolve 1-azabenzanthrone in 100mL of 60 In a mixed solution of % (volume) methanol and 70% (volume) ethanol (volume ratio of 6:1), dissolve dichlorobis(dimethylsulfoxide) platinum (II) in 50mL of water and 100% (Volume) In the mixed solution of methanol (volume ratio 3:2), the two solutions are mixed, 10mL dimethyl sulfoxide is added to the mixed solution, reacted at 90°C for 36 hours, concentrated and evaporated to remove most of the solvent (solvent added 85% of the amount), cooled to room temperature and stood still, and the reddish-brown target product monochlorine one (dimethyl sulfoxide) one (1-azabenzanthrone) platinum (II) was precipitated (yield 75 %).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com