rgd peptide-modified carboline hexahydropyrazine-1,4-dione, its preparation method, antithrombotic effect and application
A technology of hexahydropyrazine and antithrombotic drugs, which is applied in the field of biomedicine and can solve the problems of no antithrombotic compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
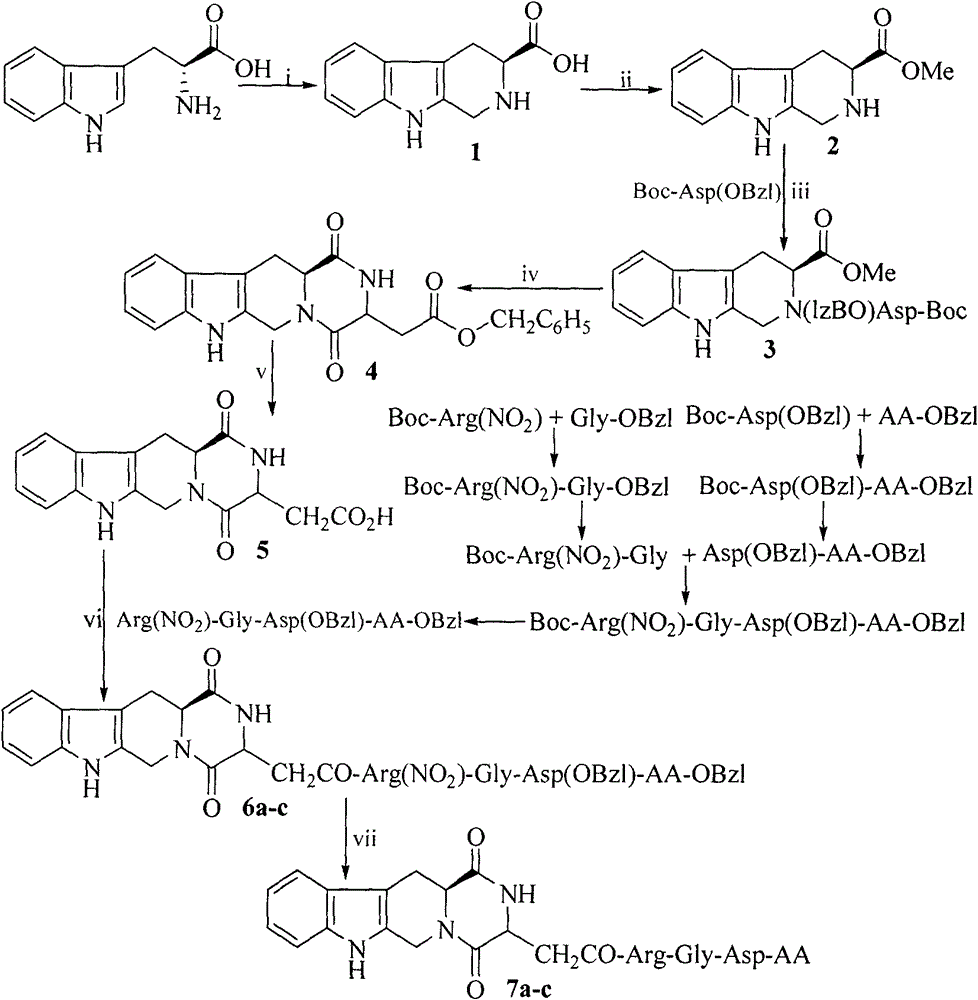
[0026] Example 1 Preparation of (3S)-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid (1)
[0027] Put 400ml water in a 500ml eggplant bottle and slowly add 0.2ml concentrated sulfuric acid. Add 5.0 g (24.5 mmol) of L-tryptophan to the obtained dilute sulfuric acid aqueous solution, and ultrasonically shake until the L-tryptophan is completely dissolved. 7.5 ml of 40% formaldehyde solution was added to the solution, and the reaction mixture was stirred at room temperature for 6 hours. TLC monitored the disappearance of L-tryptophan, and the reaction was terminated. Concentrated ammonia water was slowly added dropwise to the reaction solution, the pH of the reaction mixture was adjusted to 6, and it was allowed to stand for 0.5 hour. The precipitate was filtered under reduced pressure, washed with water, the filtered colorless solid was spread flat in a petri dish, and air dried to obtain 5.14 g (97%) of the title compound. ESI-MS(m / e): 217[M+H] + .
Embodiment 2
[0028] Example 2 Preparation of (3S)-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid methyl ester (2)
[0029] Add 50ml methanol to a 100ml eggplant bottle, cool to below 0℃ with an ice-salt bath, add 6ml (87mmol) SOCl dropwise with stirring 2 After 10 minutes of dropping, add 5.0 g (23.1 mmol) (3S)-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid, remove the ice bath, and stir overnight at room temperature. The reaction is stopped after TLC monitors the disappearance of the raw materials, and the water pump depressurizes the methanol and excess SOCl 2 , The residue is dissolved in methanol again, the circulating water vacuum pump is drained, and this is repeated three times (10ml×3), the residue is added with ether, and the circulating water vacuum pump is drained, and repeated three times (10ml×3). Add 20ml water and 20ml ethyl acetate to the residue, and add saturated NaHCO 3 The pH of the solution was adjusted to 7-8, the layers were separated, the ethyl acetate layer was col...
Embodiment 3
[0030] Example 3 Preparation of (3S)-N-[Boc-(OBzl)aspartyl]-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid methyl ester (3)
[0031] Dissolve 1.61g (5mmol) Boc-Asp (OBzl) in 50ml of anhydrous dichloromethane, add 742mg (6mmol) HOBt and 1.42g (6mmol) DCC under ice bath. After stirring for 5-10 minutes, the reaction solution appears turbid, add 1.15g (5mmol) (3S)-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid methyl ester, remove the ice bath after 30 minutes. The reaction was carried out at room temperature for 12 hours, TLC showed that the starting material point disappeared, the reaction was stopped, DCU was filtered off, the filtrate was concentrated to dryness under reduced pressure at 30°C, and the residue was dissolved in ethyl acetate. Then washed with saturated sodium bicarbonate aqueous solution, saturated sodium chloride aqueous solution, 5% potassium hydrogen sulfate aqueous solution, saturated sodium bicarbonate aqueous solution and saturated sodium chloride aqueou...
PUM
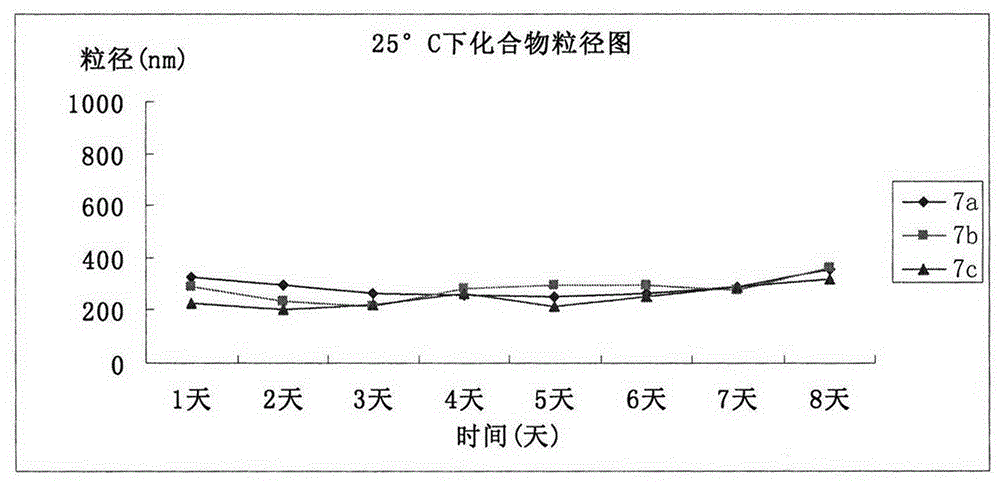
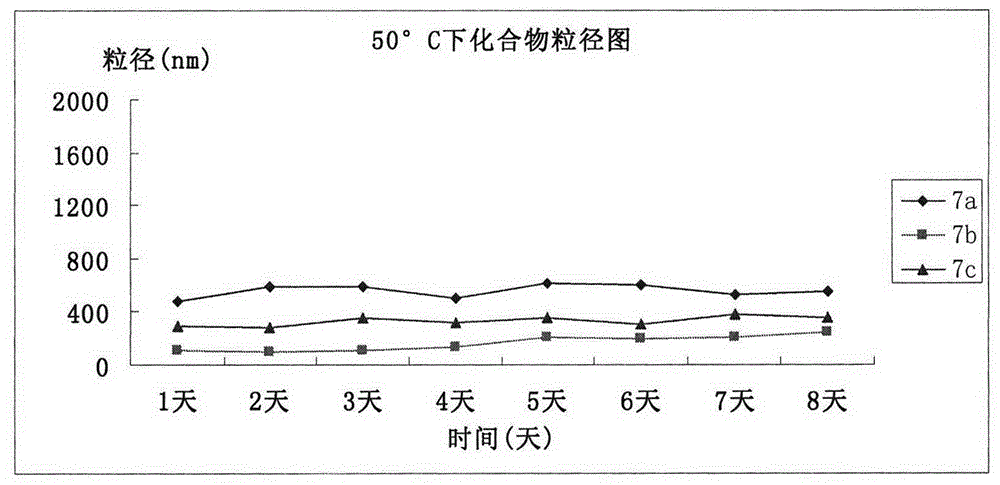
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com