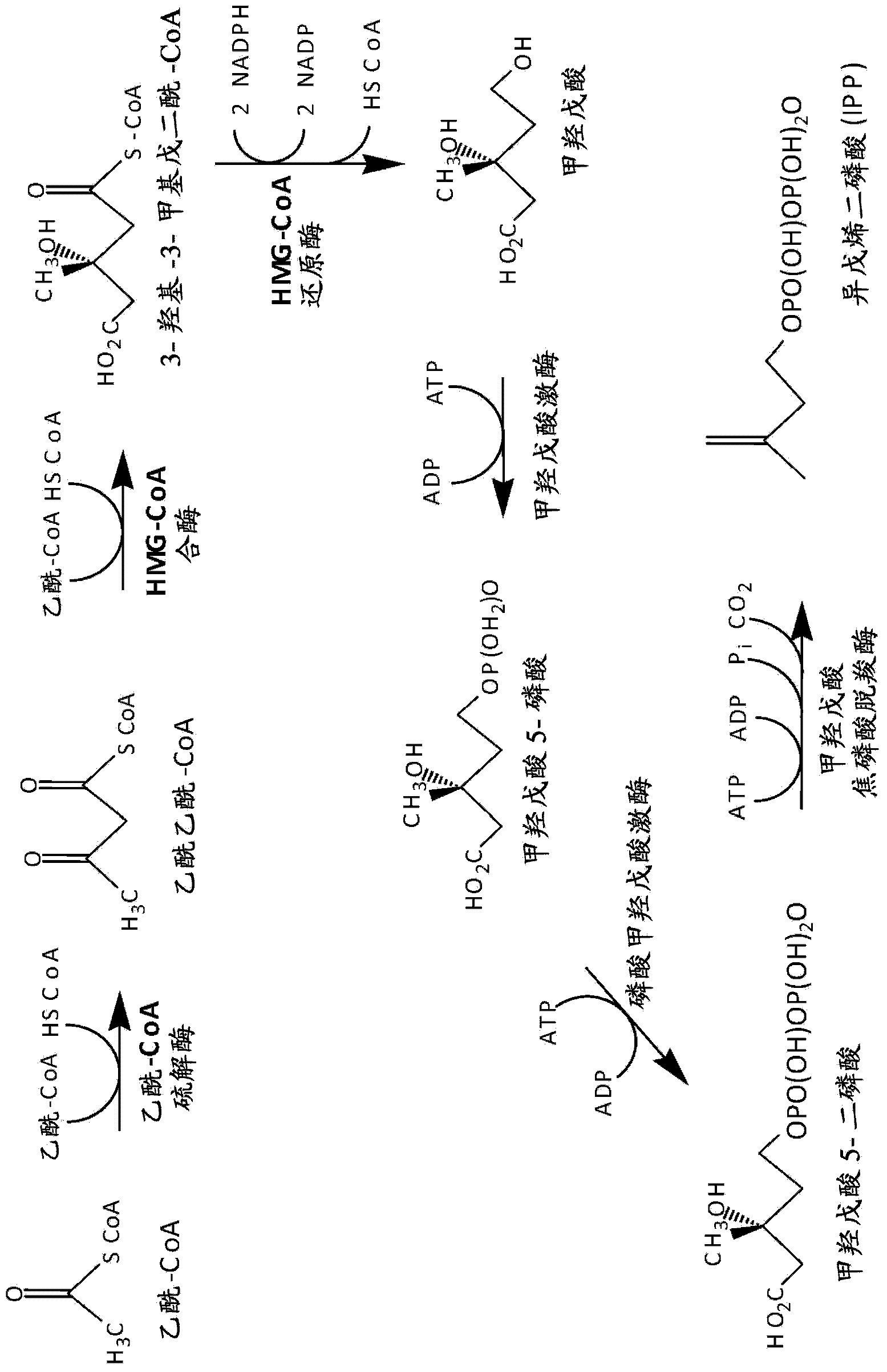
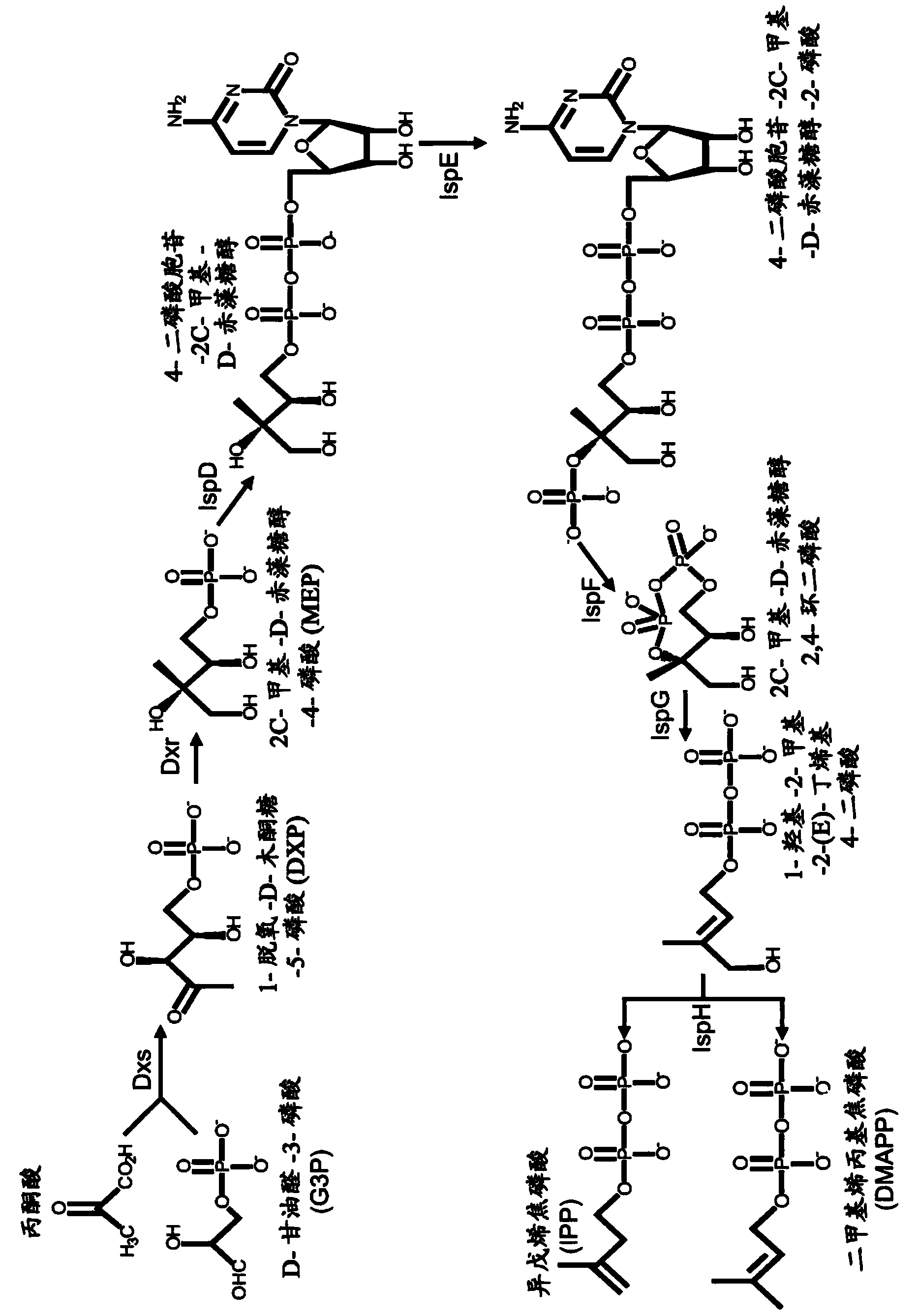
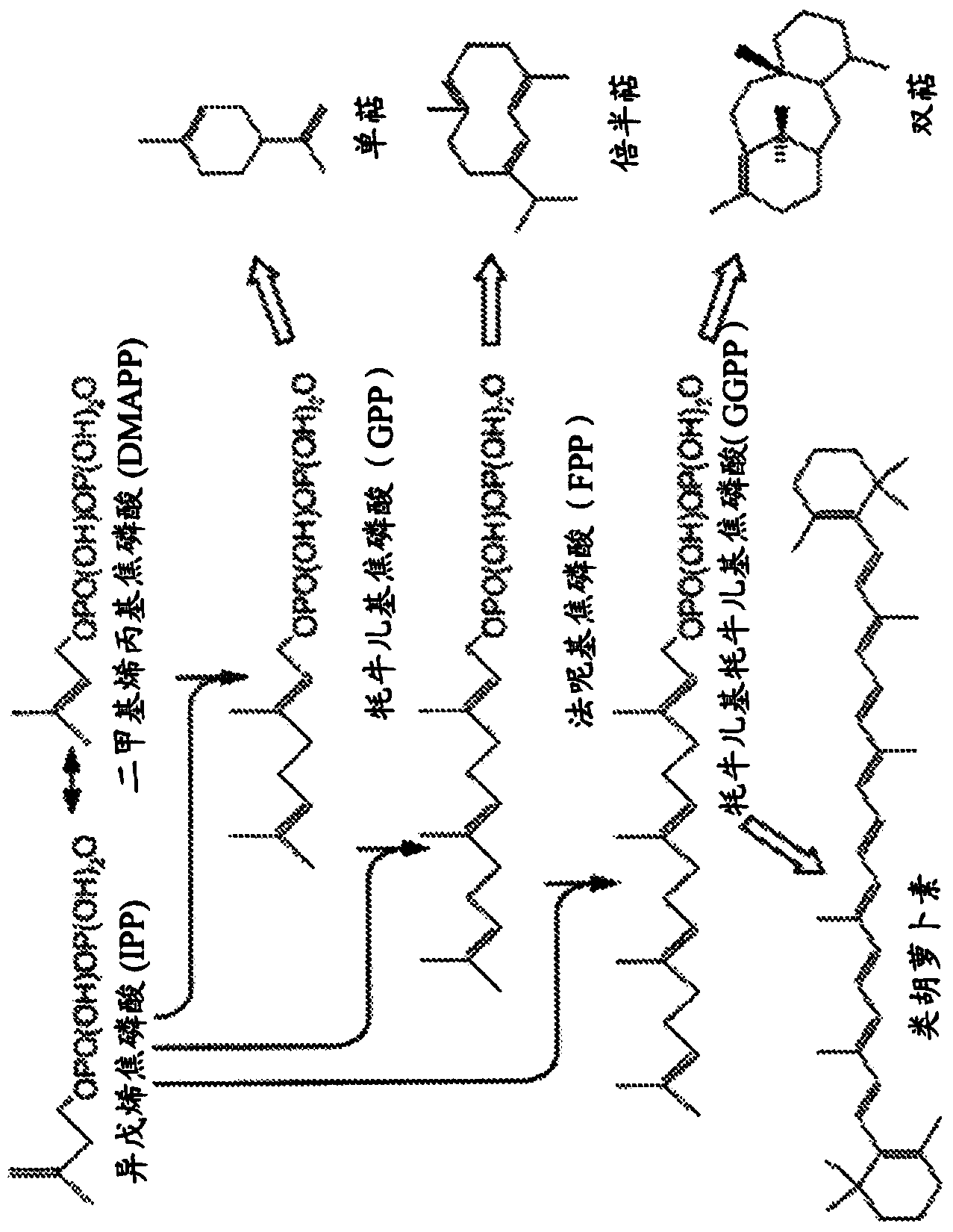
Gel-encapsulated microcolony screening
A technology of encapsulation and hydrogel, applied in the determination/inspection of microorganisms, immobilization on/in organic carriers, instruments, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0272] 7.2 Example 2: Encapsulation of cells
[0273] This example describes exemplary methods for encapsulating cells into hydrogels and screening the encapsulated cells for recombinant production of one or more water-immiscible compounds.
[0274] 7.2.1 Fabrication of microfluidic systems
[0275] Microfluidic devices composed of the elastomeric polymer poly(dimethylsiloxane) (PDMS) and containing at least two channels interconnected at T-junctions are prepared using etched wafer substrates (e.g., by photolithography ) as die manufactured.
[0276] Briefly, wafer substrates were prepared by rinsing the wafer with acetone and isopropanol. A photoresist such as SU8-3000 photoresist (Microchem, Newton, MA) is applied to the wafer by spin coating. This photoresist coating is then selectively irradiated with UV light through a mask designed to allow exposure of the photoresist in a selected pattern (e.g., a pattern involving two channels interconnected at T-junctions). Floor....
Embodiment 3
[0288] 7.3 Example 3: Particle Analysis and Sorting
[0289] This example describes exemplary methods for the analysis and sorting of hydrogel particles comprising cells that produce water-immiscible compounds.
[0290] Place a 70 μm BD cell strainer on a 50 ml conical tube. The culture containing the encapsulated cells was applied to the center of the filter with a 5 ml pipette. The particles were washed off the membrane with two 1 ml aliquots of PBS, centrifuged at 100 g for 30 s, resuspended in 1 ml PBS and centrifuged again at 100 g for 30 s. The filtered culture was then added in aliquots to 10 μm Partec filters and centrifuged at 100 g for 90 seconds. Add 1 ml PBS to the filter cake of each filter and resuspend the cake by pipetting up and down. Another 1 ml of PBS was added to the suspension and centrifuged at 100 g for 90 seconds.
[0291] Prepare Nile Red staining solution (2 ml / sample), as needed, by adding 200 µl of Nile Red stock solution (100 µg / ml in EtOH) pe...
Embodiment 4
[0297] 7.4 Example 4: Identification and Selection of Farnesene-Producing Cells
[0298] This example demonstrates the sensitivity and fidelity of detection of heterologous water-immiscible compounds in recombinant yeast strains engineered to produce low to high levels of farnesene. A series of yeast strains produced according to the method described in Example 1 and representing a broad range of farnesene production were encapsulated and sorted according to the methods described in Examples 2 and 3, respectively. Nile red was used as a detection agent. Farnesene levels detected by the skin screening method were compared to levels detected by standard measurements including 2-liter fermentor yield, Nile Red 96-well vibrating plate, and farnesene flux.
[0299] The Nile Red 96-well shaker plate assay was performed as follows. For each strain, pick a single colony from the agar plate into a 1.1 ml 96-well plate containing 360 μl of Bird Seed medium (BSM), 2% sucrose, 0.25N+CRB...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com