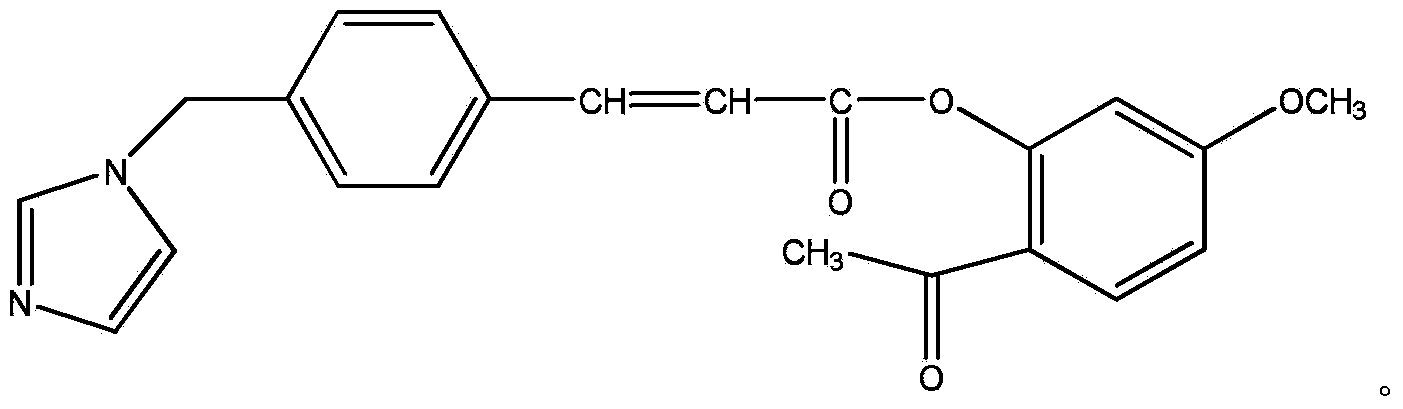
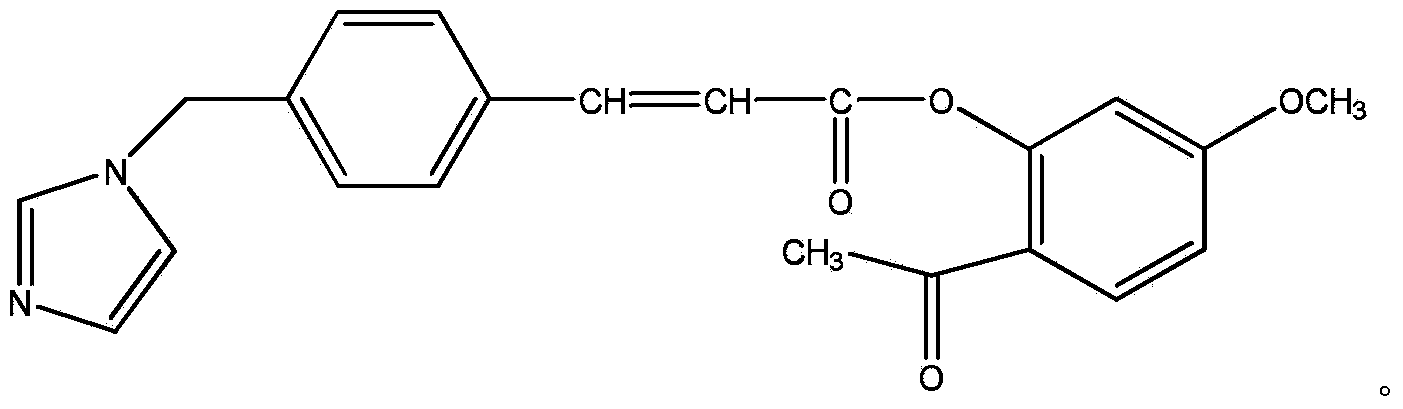
Conjugate of paeonol and ozagrel, pharmaceutical composition and medical application of conjugate
A technology of paeonol and its composition, applied in the field of medicinal chemistry, can solve the problems of easy decomposition, poor drug compliance of patients, unstable aqueous solution, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
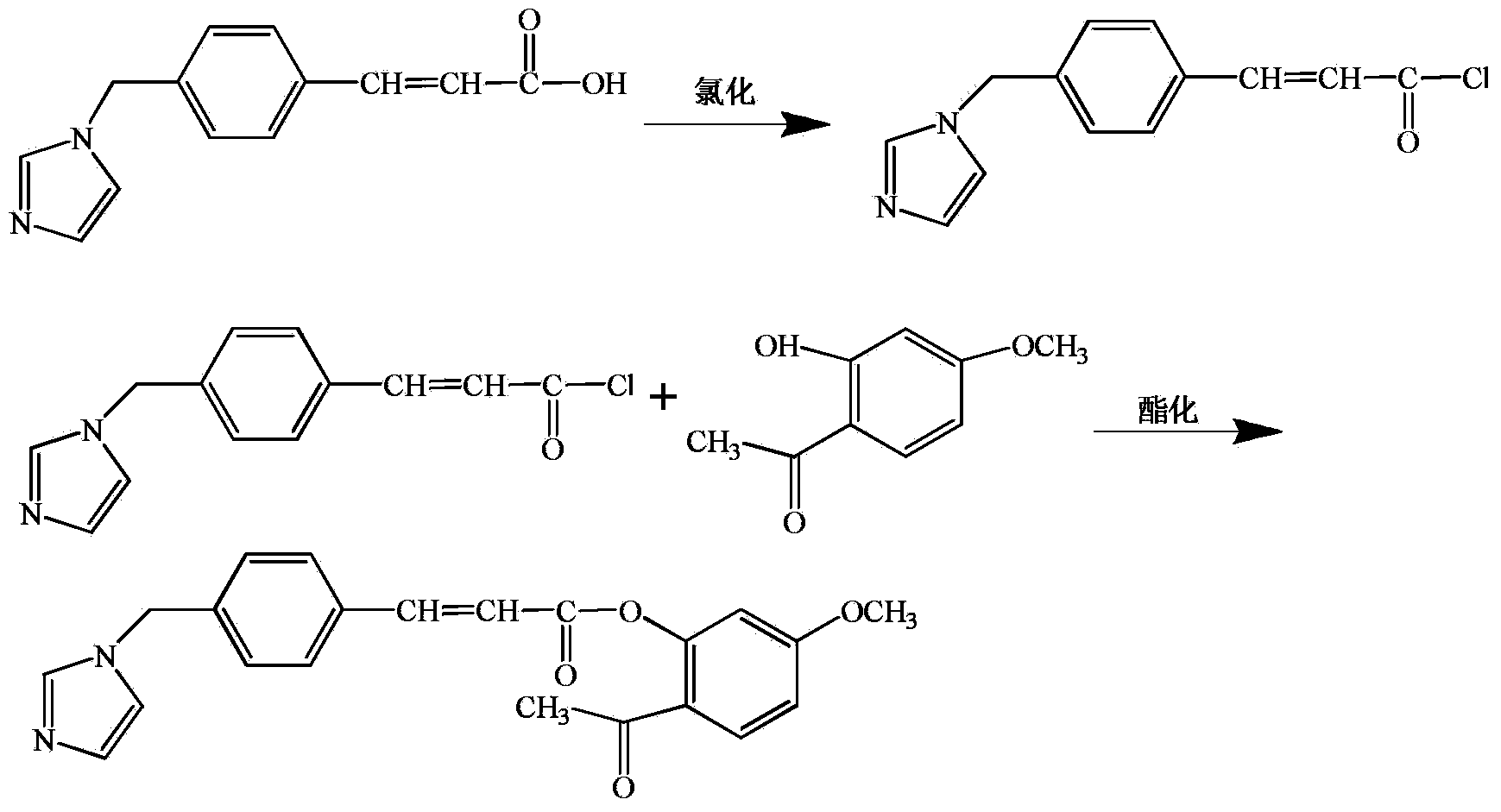
[0019] The preparation method of the compound of the present invention is as follows:
[0020]
[0021] The compounds of the present invention can be made into common pharmaceutical preparations by adding pharmaceutically acceptable carriers, such as tablets, capsules, powders, syrups, liquids, suspensions, injections, spices, sweeteners, liquid or solid fillers can be added Or pharmaceutically acceptable pharmaceutical excipients such as diluents.
[0022] The clinical administration of the compound of the present invention can be oral administration, injection and the like.
[0023] Pharmacological tests prove that the compound of the present invention has the effect of preventing or treating cardiovascular and cerebrovascular diseases caused by platelet aggregation, especially can treat diseases such as thrombotic heart and brain ischemia or infarction.
[0024] Below is the pharmacological test and the result of compound of the present invention:
[0025] Experimental...
Embodiment 1
[0036] 1.1 Synthesis of (E)-3-(4-((1H-imidazolyl-1-yl)methyl)phenyl)acryloyl chloride
[0037] Reaction formula:
[0038]
[0039] Reaction steps:
[0040] Ozagrel (6.84g, 0.03mol), CH 2 Cl 2 (60ml) were mixed, 5 drops of DMF was added, and SOCl was added dropwise under stirring at room temperature 2 (15ml) in CH 2 Cl 2 The solution in (30ml) drips in about half an hour, and after dripping, the external temperature is heated to 70 degrees Celsius, and refluxes for 2.5 hours. The reaction solution was evaporated to dryness under reduced pressure to remove SOCl2 , CH 2 Cl 2 , the residue plus CH 2 Cl 2 (20ml) beating, suction filtration to obtain 7.51g of light yellow solid, yield 88.5%.
[0041] 1.2 Synthesis of (E)-2-acetyl-5-methoxyphenyl-3-(4-((1H-imidazolyl-1-yl)methyl)phenyl)acrylate
[0042] Reaction formula:
[0043]
[0044] Reaction steps:
[0045] Paeonol (3.67g, 0.022mol), CH 2 Cl 2 (60ml) and triethylamine (7.5ml) were mixed, and the product fro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com