Pharmaceutical composition used for preventing and treating non-alcoholic fatty liver disease and application thereof
A non-alcoholic, composition technology, applied in the direction of drug combination, pharmaceutical formula, plant raw materials, etc., to achieve significant synergistic effect, significant effect, good curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Preparation of tablets comprising a combination of curcumin and berberine
[0039] (1) The weight percent of curcumin and berberine in the composition can be, but not limited to:
[0040]
[0041] (2) Select the ratio of curcumin: berberine = 67:33, 60:40, 50:50, 40:60, 33:67 to form the tablet formula in Table 1 below and prepare corresponding tablets:
[0042] Table 1.
[0043]
[0044]
[0045] Tablet preparation method:
[0046] ①Weigh the prescribed amount of curcumin (purchased from Zhengzhou Xingyao Technology Co., Ltd.) and berberine hydrochloride (purchased from Sichuan Weikeqi Biotechnology Co., Ltd.) respectively, pass through 100 mesh sieves, and then mix according to the method of equal increments for later use ;
[0047] ②According to the tablet prescription in Table 1, weigh the prescription amount of other auxiliary materials except 5% povidone K-30 and magnesium stearate and mix them for subsequent use;
[0048] ③ Add an appropriate amoun...
Embodiment 2
[0054] Preparation of turmeric extract containing curcumin as the main medicinal component and berberine as the main Tablets of the composition of the Coptis chinensis extract as the medicinal ingredient
[0055] (1) Preparation of turmeric extract with curcumin as the main active ingredient and berberine as the main active ingredient in Coptis chinensis extract
[0056] Turmeric extract with curcumin as the main medicinal ingredient: crush 50kg of dried rhizomes of turmeric (produced in Sichuan), add 75% (volume percentage) ethanol aqueous solution at a liquid-to-solid ratio of 10:1, soak at room temperature for 24 hours, and pour out the ethanol aqueous solution , re-add new 75% (volume percent) ethanol aqueous solution and soak for 24 hours again, repeat 3 times, combine the ethanol aqueous solution, concentrate under reduced pressure to remove the ethanol aqueous solution, and obtain turmeric extract (powder, dry Back weight: 8.5kg), according to high performance liqui...
Embodiment 3
[0082] Experiments on pharmacological activity of compositions comprising curcumin and berberine
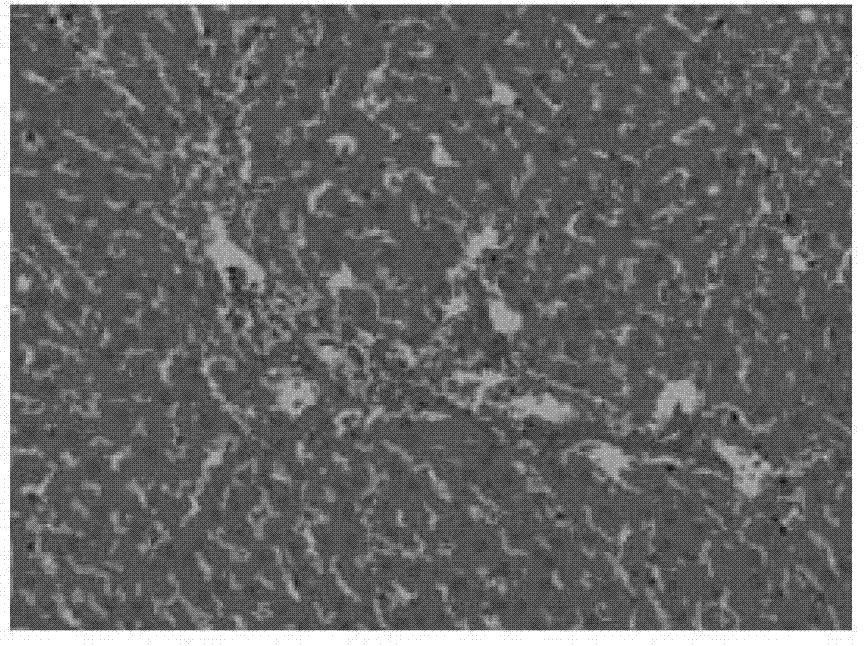
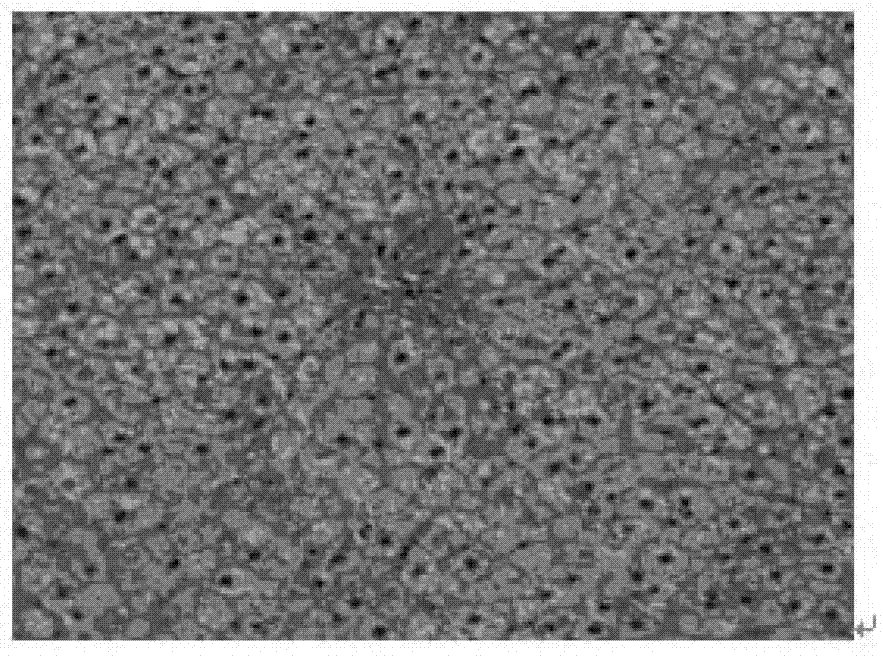
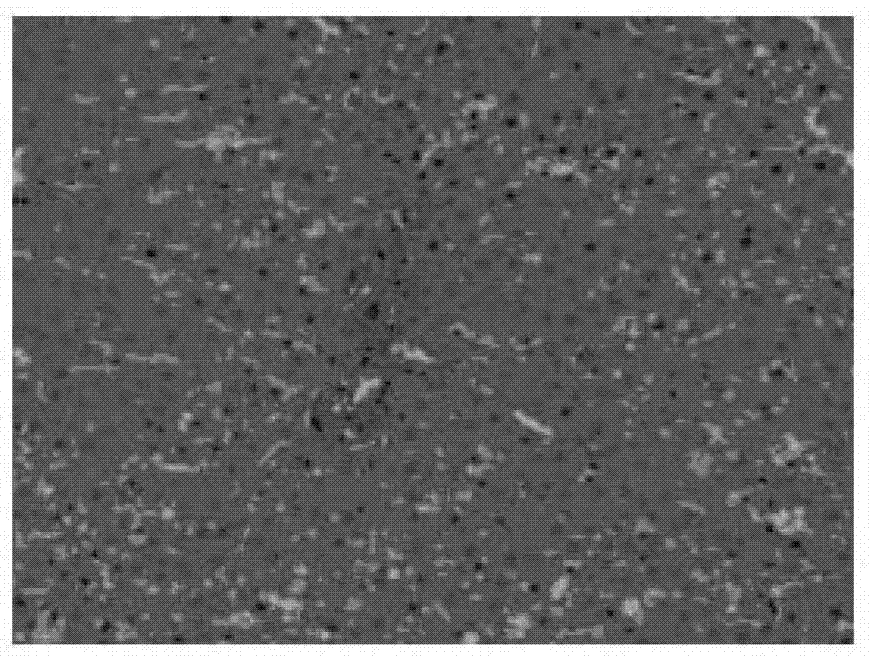
[0083] ①Therapeutic effect on fatty liver:
[0084] Experimental animals: SD rats, 120, ♂, clean grade, initially 6-8 weeks old, 100-150g. Feed with normal or high-fat diet. Fatty liver model was induced by feeding high-fat diet for 10 weeks.
[0085] Experimental grouping: normal rat group (10 rats), fatty liver model group (10 rats), fatty liver model curcumin intervention group (100mg / kg.d) (10 rats), fatty liver model berberine intervention group (100mg / kg.d) (10 rats), fatty liver model composition intervention group ((curcumin + berberine) mg / kg.d: (100+100), (50+50), (100+50), (50+100), (90+60), (60+90), (120+40), (40+120) mg / kg.d) (10 rats in the composition intervention group for each dose). Each group was administered for 10 weeks and administered by intragastric administration.
[0086] Among them, curcumin was purchased from Zhengzhou Xingyao Technology Co., L...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com