Synthesis method of 8-hydroxyquinaldine
A synthetic method and the technology of hydroxyquinaldine, which are applied in the field of synthesis of 8-hydroxyquinaldine, can solve the problems of high toxicity, limited industrial scale production, great harm to operators and production environment, and achieve low raw material cost and high process efficiency. simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
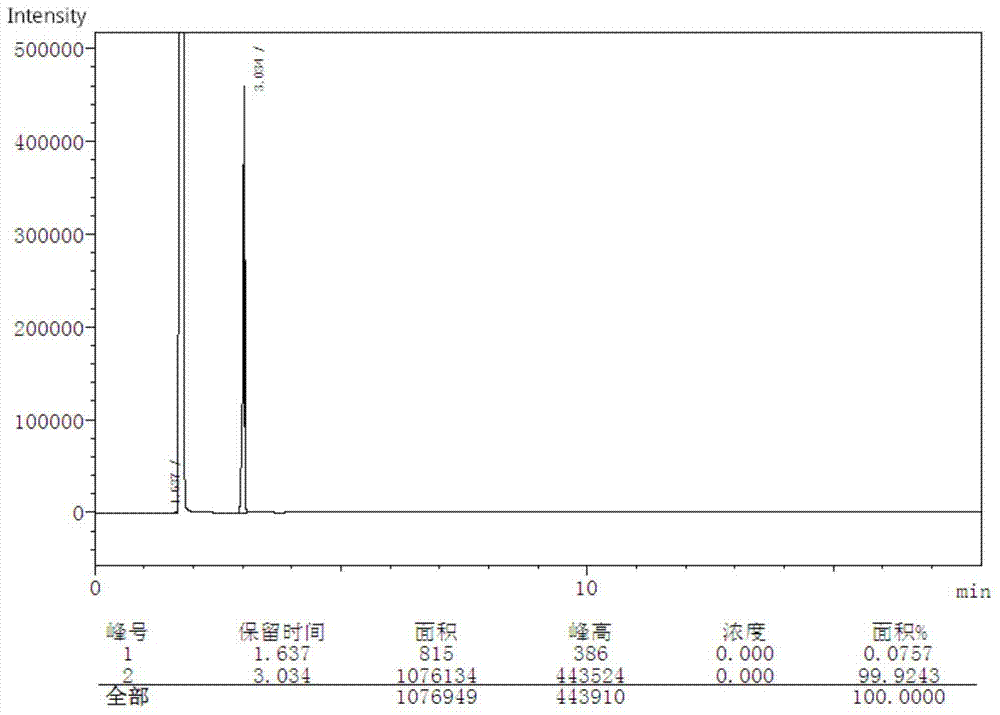
[0030] Example 1: In a 2L glass reactor, add 109g of o-aminophenol, 30g of o-nitrophenol, 500mL of 30% hydrochloric acid and 30g of glacial acetic acid in sequence, and after fully dissolving them at 60°C, slowly add three After adding 90g of metaldehyde, keep it warm for 6 hours, pass 0.5MPa water vapor to carry out steam distillation for 3 hours, recover o-nitrophenol, cool the remaining liquid in the reaction kettle to room temperature, add 30% NaOH to neutralize to pH=6.8, Filtration, washing with hot water and drying to obtain 100 g of a yellow-brown solid crude product with a yield of 63% and a purity of 90.0% (GC detection, see Figure 5 ).
[0031] The above crude product was subjected to vacuum distillation (collecting fractions at 185-190°C / 25kPa) and recrystallization from 20% alcohol aqueous solution to obtain 65 g of white solid with a yield of 65% and a purity of 98.7% (GC detection, see Figure 6 ).
Embodiment 2
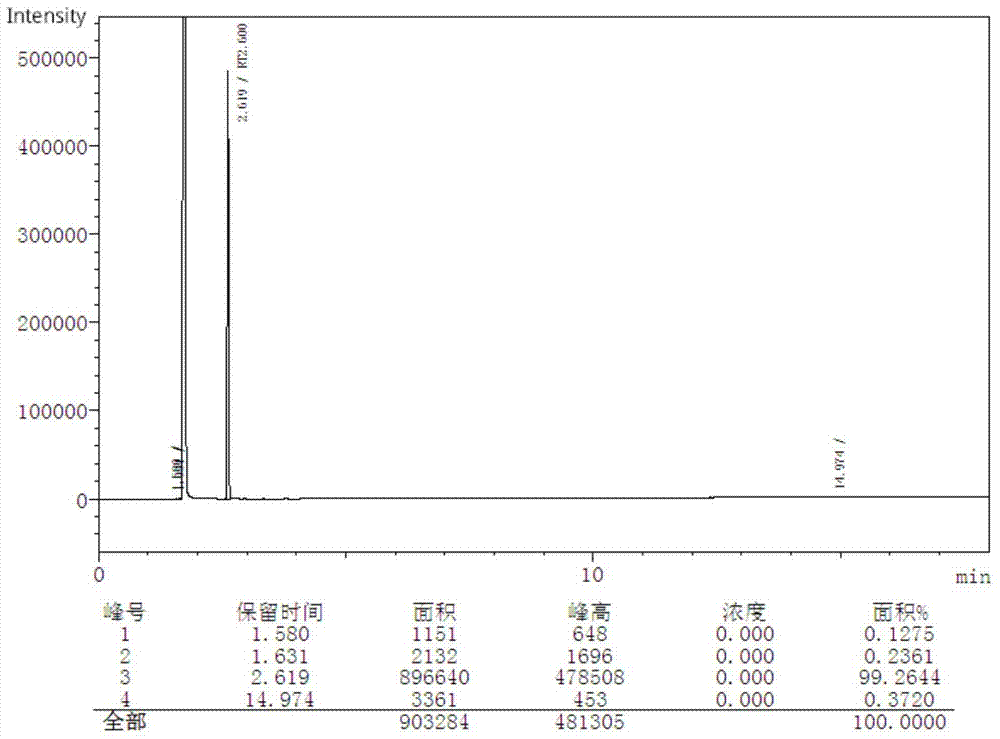
[0032] Example 2: In a 2L glass reactor, add 109g of o-aminophenol, 40g of o-nitrophenol, 500mL of 37% hydrochloric acid and 45g of glacial acetic acid in sequence. After fully dissolving them at 80°C, slowly add three Metaldehyde 105g, after adding, keep warm for 4 hours, pass 0.5MPa water vapor to carry out steam distillation for 3 hours, recover o-nitrophenol in the distillate, cool the remaining liquid in the reaction kettle to room temperature, neutralize with liquid caustic soda to pH=6 , sodium carbonate is neutralized again until no bubbles are produced, filtered, washed with hot water, and dried to obtain 109 g of brown solid crude product, with a yield of 69% and a purity of 86.0% (GC detection, see Figure 7 ).
[0033] The above crude product was subjected to vacuum distillation (185-190°C / 25kPa fraction was collected) and recrystallized from 30% alcohol aqueous solution to obtain 66.5g of white solid with a yield of 61% and a purity of 97.6% (GC detection, see F...
Embodiment 3
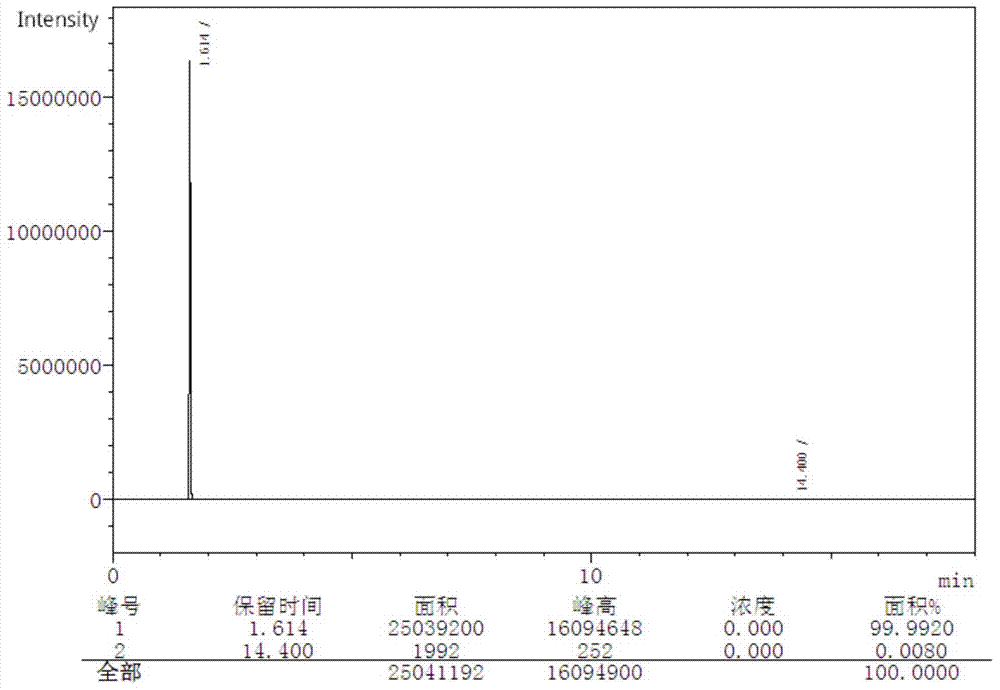
[0034] Example 3: In a 2L glass reactor, add 109g of o-aminophenol, 50g of o-nitrophenol, 500mL of 19% hydrochloric acid and 45g of glacial acetic acid in sequence, and after fully dissolving them at reflux temperature, slowly add three Metaldehyde 120g, after adding, keep warm for 2 hours, pass 0.5MPa water vapor to carry out steam distillation for 3 hours, recover o-nitrophenol from the distillate, cool the remaining liquid in the reaction kettle to room temperature, neutralize with ammonia water to pH=6.8, filter , washed with hot water to obtain 116g of brown solid crude product, yield 73%, purity 82.1% (GC detection, see Figure 9 ).
[0035] The above crude product was subjected to vacuum distillation (185-190°C / 25kPa fraction was collected) and recrystallized from 30% alcohol aqueous solution to obtain 71.5g of white solid with a yield of 62% and a purity of 99.7% (GC detection, see Figure 10 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com