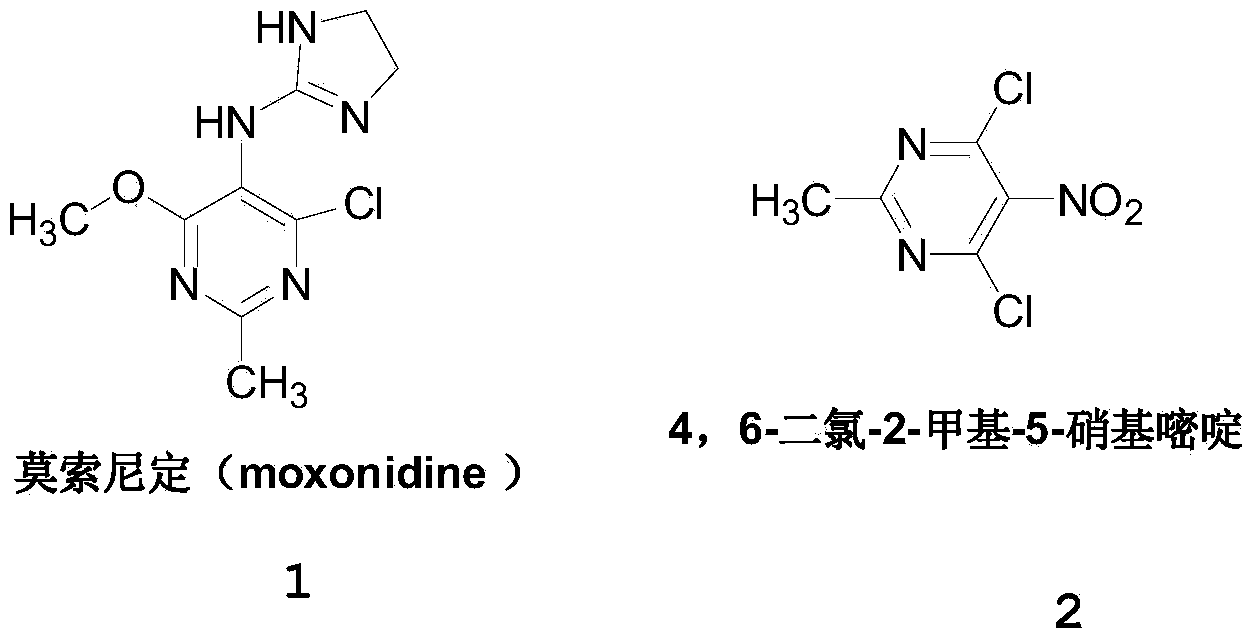
Method for preparing 4, 6-dichloro-2-methyl-5-nitro pyrimidine
A technology of nitropyrimidine and methyl group, applied in the field of pharmaceutical chemistry, can solve the problems of highly toxic substances, ecological environment pollution, etc., and achieve the effects of less waste water, strong operability, and short technical process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
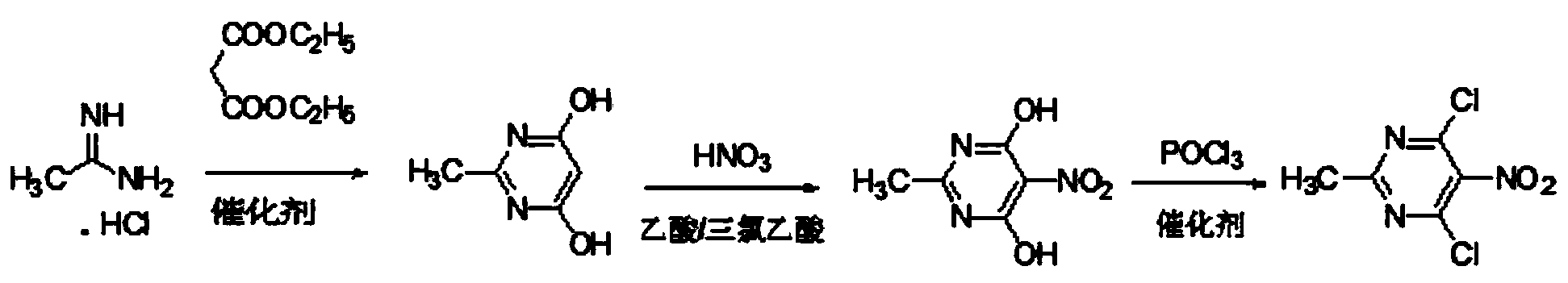
[0025] Preparation of 4,6-dihydroxy-2-methylpyrimidine
[0026] Add acetamidine hydrochloride (47.3 g, 0.50 mol), diethyl malonate (76.5 g, 0.52 mol) and methanol (200 ml) into a 1.0L reaction flask, add 30% (solute mass fraction) under stirring at room temperature Sodium methoxide-methanol solution (275.5 g, 1.53 moles), stirred and heated to reflux for 3 hours, TLC followed the reaction, after the reaction was complete, cooled, filtered, washed with water and methanol, then the filter cake was dissolved in 350 ml of water, concentrated Adjust pH=2 with hydrochloric acid, a white precipitate precipitates out, let stand, filter, wash with water, and dry to obtain off-white solid 4,6-dihydroxy-2-methylpyrimidine (57.5g), yield 91.2% (acetamidobenzene hydrochloride count),
[0027] M.p.338-345℃, 1 H NMR: (400Hz, DMSO-d 6 ),δ(ppm):11.75(s,2H,-OH),4.95(s,1H,CH-),2.21(s,3H,CH 3 -).;
[0028] (1) Preparation of 4,6-dihydroxy-2-methyl-5-nitropyrimidine
[0029] Add the 4,6-dihy...
example 2
[0035] Preparation of 4,6-dihydroxy-2-methylpyrimidine
[0036]Add acetamidine hydrochloride (47.3 g, 0.50 mol), diethyl malonate (76.5 g, 0.52 mol) and ethanol (150 ml) into a 1.0L reaction flask, add 20% (solute mass fraction) under stirring at room temperature Sodium ethoxide-ethanol solution (476.0 g, 1.42 moles), stirred and heated to reflux for 4 hours, TLC followed the reaction, after the reaction was complete, cooled, filtered, washed with water, washed with methanol, then the filter cake was dissolved in 450 ml of water, sulfuric acid Adjust the pH to 1, and a white precipitate precipitates out, let stand, filter, wash with water, and dry to obtain off-white solid 4,6-dihydroxy-2-methylpyrimidine (56.6g), with a yield of 89.8% (calculated as acetamidobenzene hydrochloride ).
[0037] (1) Preparation of 4,6-dihydroxy-2-methyl-5-nitropyrimidine
[0038] Add the 4,6-dihydroxy-2-methylpyrimidine (12.6g, 0.10mol), trichloroacetic acid (12.3g, 0.075mol) and acetic acid (1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com