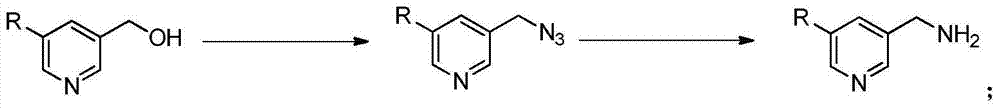
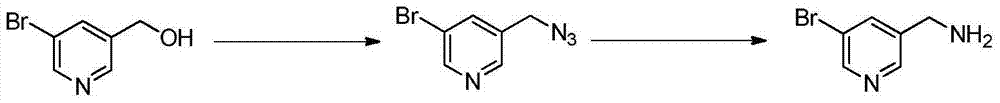
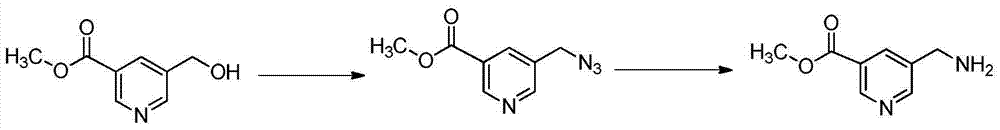
Preparation method of 5-aminomethyl pyridine derivative
A technology of aminomethylpyridine and hydroxymethylpyridine, which is applied in the field of preparation of 5-aminomethylpyridine derivatives, can solve the problems of low yield and achieve the effects of high yield, mild conditions and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 15
[0019] The preparation of embodiment 15-aminomethyl-3-bromopyridine
[0020] The preparation of 5-aminomethyl-3-bromopyridine adopts the following process:
[0021] (1) Preparation of 5-azidomethyl-3-bromopyridine: Add 1.00g of 5-hydroxymethyl-3-bromopyridine, 2.63g of diphenylphosphoryl azide and 10mL of tetrahydrofuran into a three-neck flask; nitrogen protection , the reaction system was cooled to below 10°C, and 0.89g of 1,8-diazabicyclo[5.4.0]undec-7-ene was added dropwise to the reaction liquid, and reacted at room temperature for 12h. The volatiles were removed by concentration under reduced pressure, and 0.98 g of 5-azidomethyl-3-bromopyridine was obtained by column chromatography.
[0022] (2) Preparation of 5-aminomethyl-3-bromopyridine: Dissolve 0.98g of 5-azidomethyl-3-bromopyridine in 20ml of THF and 2ml of water, add 2.41g of triphenylphosphine under ice-water bath, 5 The reaction was carried out at ℃ for 16 h; extracted with ethyl acetate, concentrated, and 0....
Embodiment 25
[0027] The preparation of embodiment 25-aminomethyl-3-bromopyridine
[0028] The preparation of 5-aminomethyl-3-bromopyridine adopts the following process:
[0029] (1) Preparation of 5-azidomethyl-3-bromopyridine: Add 0.1mol 5-hydroxymethyl-3-bromopyridine, 0.1mol diphenylphosphoryl azido and 50mL toluene into a three-neck flask; nitrogen protection , the reaction system was cooled to below 10°C, 0.01mol tetrabutylammonium bromide was added dropwise to the reaction solution, and the reaction was refluxed for 8h. Concentrate under reduced pressure to remove volatiles, and obtain 5-azidomethyl-3-bromopyridine by column chromatography.
[0030] (2) Preparation of 5-aminomethyl-3-bromopyridine: Dissolve 0.05mol of 5-azidomethyl-3-bromopyridine in 20ml of methanol and 2ml of water, add 5mmol of palladium carbon in an ice-water bath, and place at -5°C Reaction for 30h; extraction with ethyl acetate, concentration, and column chromatography to obtain the product.
Embodiment 35
[0031] The preparation of embodiment 35-aminomethyl-3-bromopyridine
[0032] The preparation of 5-aminomethyl-3-bromopyridine adopts the following process:
[0033] (1) Preparation of 5-azidomethyl-3-bromopyridine: Add 0.1mol 5-hydroxymethyl-3-bromopyridine, 0.3mol diphenylphosphoryl azido and 50mL N,N- Dimethylformamide; under nitrogen protection, cool the reaction system below 10°C, add 0.1mol tetrabutylammonium iodide, 0.1mol N,N-diisopropylethylamine, 0.05mol p-toluenesulfonyl chloride , 0.05mol2,4,6-trimethylbenzenesulfonyl chloride was added dropwise to the reaction solution, and reacted at room temperature for 16h. Concentrate under reduced pressure to remove volatiles, and obtain 5-azidomethyl-3-bromopyridine by column chromatography.
[0034] (2) Preparation of 5-aminomethyl-3-bromopyridine: Dissolve 0.05mol 5-azidomethyl-3-bromopyridine in 20ml of N,N-dimethylformamide and 2ml of water, and add 5mmol of aluminum chloride was reacted at room temperature for 8h; ext...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com