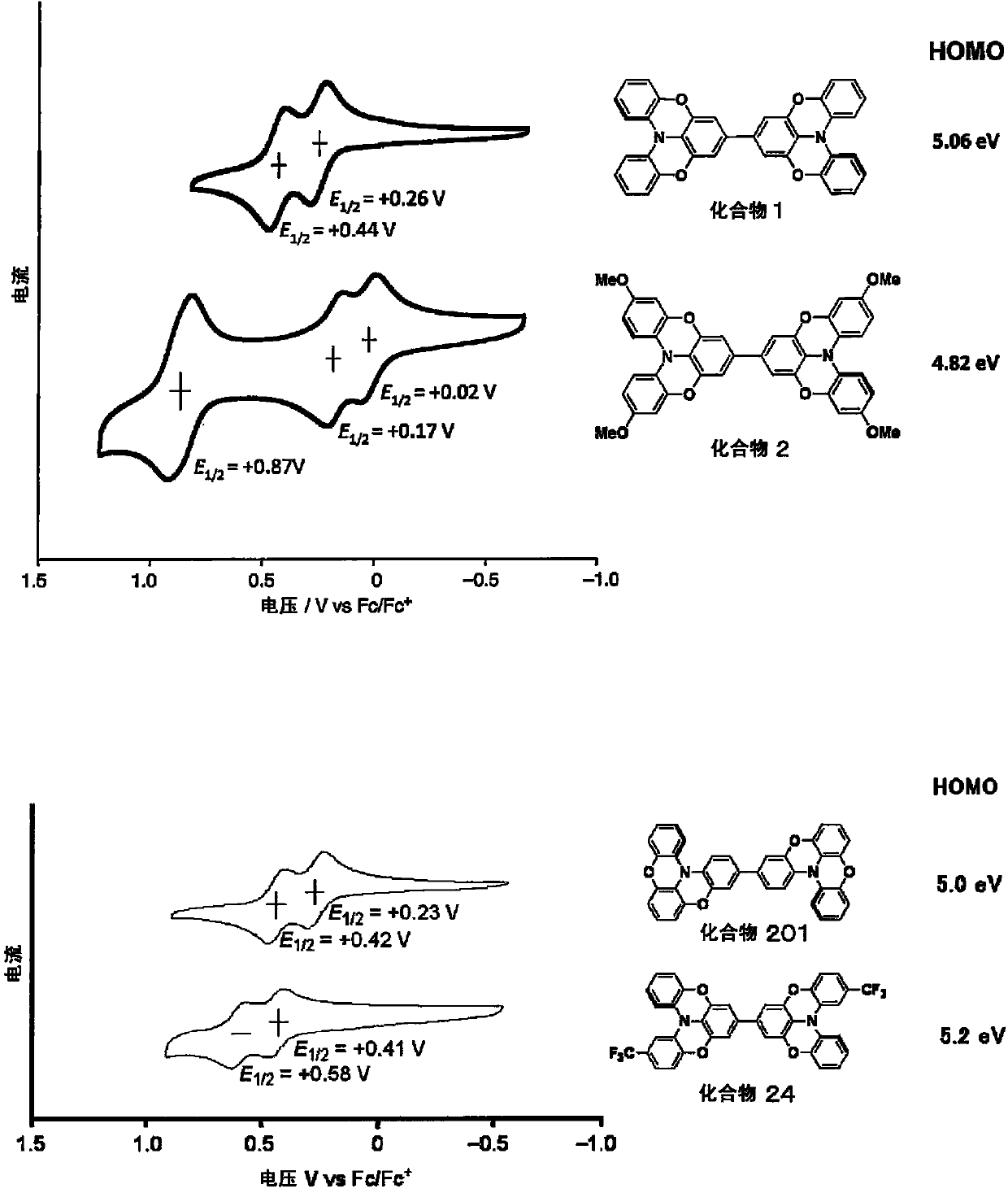
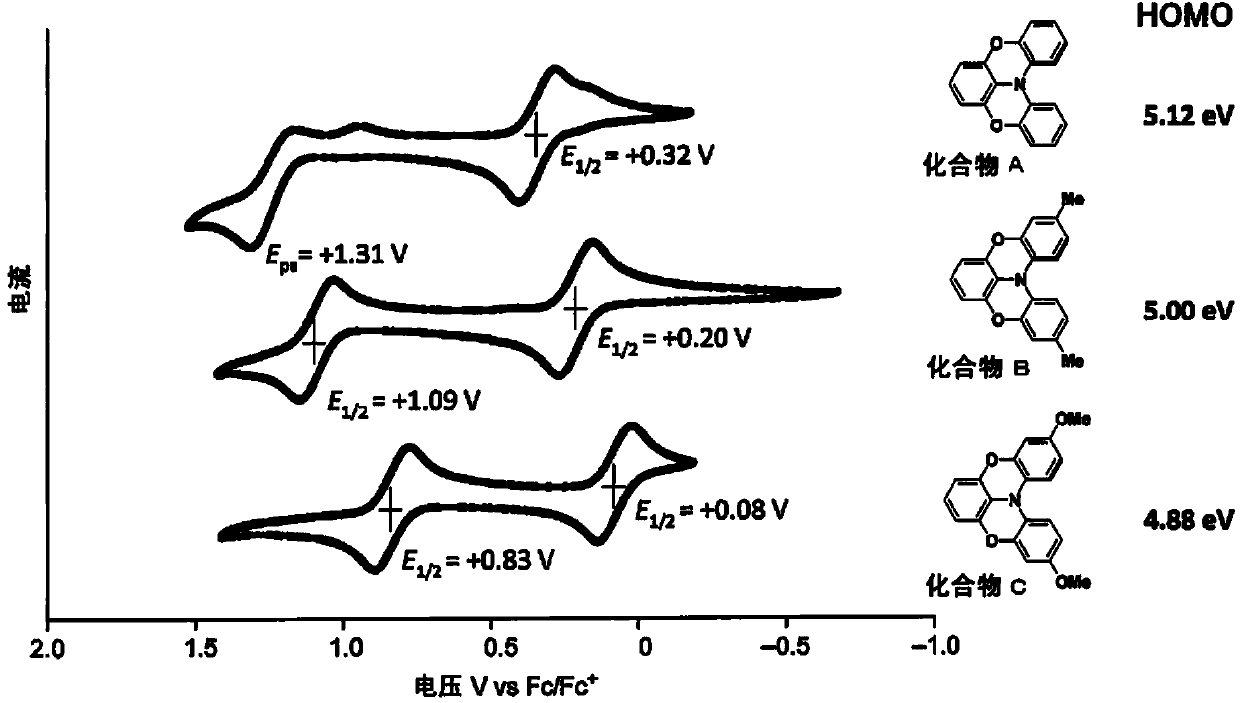
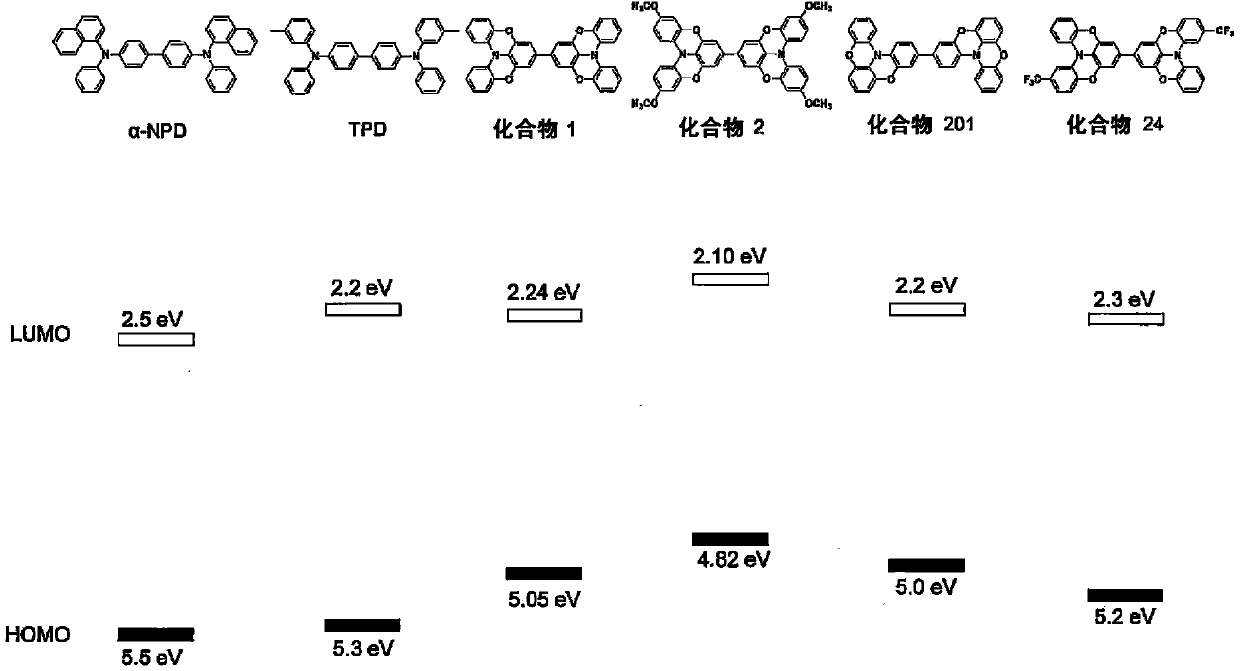
Novel compound, charge transport material, and organic device
A compound and bonding technology, which is applied in the direction of electric solid-state devices, electric components, luminescent materials, etc., can solve the problems of unrecorded production of dimers of triphenylamine derivatives, and achieve the effect of suppressing power consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0297] Compound 1 was synthesized according to the following scheme.
[0298] [chemical formula 27]
[0299]
[0300] Compound 21a (19.8g, 84.6mmol), compound 23a (7.74g, 37.2mmol), K 2 CO 3 (21.5g, 156mmol), and Cu (7.82g, 123mmol) were dissolved in o-dichlorobenzene [ODCB] (100ml) and heated at 180°C for 110 hours. The reaction mixture was filtered, and the insoluble matter was washed three times with chloroform (100 ml). The filtrate was washed with water and then washed with MgSO 4Dry, filter, and concentrate under reduced pressure. Furthermore, the obtained black solid was washed with hexane to obtain Compound 24a (10.6 g, 25.4 mmol) as a white powder with a yield of 68%.
[0301] Mp: 157.5-158.5°C.
[0302] 1 H NMR (300MHz, CDCl 3 , ppm) δ7.10-6.94 (m, 4H), 6.94-6.81 (m, 6H), 3.60 (s, 6H).
[0303] 13 C NMR (75MHz, CDCl 3 , ppm): δ158.24 (dd, 1 J(C,F)=252.4, 3 J(C,F)=6.9Hz), 153.25, 136.12, 124.61, 121.10, 115.29 (dd, 2 J(C,F)=17.8, 4 J(C,F)=9.2Hz), 114...
Embodiment 2
[0321] Compound 2 was synthesized according to the following scheme.
[0322] [chemical formula 28]
[0323]
[0324] Compound 21b (20.4g, 77.2mmol), compound 23b (6.86g, 33.0mmol), K 2 CO 3 (18.2g, 132mmol), and Cu (6.80g, 107mmol) were dissolved in o-dichlorobenzene [ODCB] (90ml) and heated at 180°C for 150 hours. The insoluble material was removed by filtration with CH 2 Cl 2 (100ml) was washed three times, and the filtrate was washed with water. The obtained organic phase was washed with MgSO 4 Dry, filter, and concentrate under reduced pressure. Then utilize silica gel column chromatography (developing solvent: hexane / CH 2 Cl 2 (1 / 3), Rf=0.56) to obtain compound 24b (9.63 g, 20.1 mmol) as a white solid in 61% yield.
[0325] Mp: 96.4-97.3°C.
[0326] 1 H NMR (300MHz, CDCl 3 , ppm) δ7.05-6.90 (m, 2H), 6.83 (d, 3 J(H,H)=8.4Hz,2H), 6.46(d, 4 J(H,H)=2.7Hz,2H), 6.38(dd, 3 J(H,H)=8.7, 4 J(H, H)=2.7Hz, 2H), 3.78(s, 6H), 3.60(s, 6H).
[0327] 13 C NMR (75MHz,...
Embodiment 3
[0342] Compound 24 was synthesized according to the following scheme.
[0343] [chemical formula 29]
[0344]
[0345] Compound 21c (11.7g, 49.9mmol), compound 23c (9.19g, 44.2mmol), Pd 2 (dba) 3 · CHCl 3 (0.799g, 0.765mmol), sodium tert-butoxide (4.38g, 45.6mmol), tri-tert-butylphosphine (0.920g, 4.55mmol) were dissolved in anhydrous toluene (100ml), and stirred at 100°C for 26 hours. The insoluble matter was filtered and washed with toluene (60ml). Then, water was added to the filtrate, and extracted with toluene (50ml×3). The organic layer was washed with Na 2 SO 4 Dry, filter, and concentrate the filtrate under reduced pressure. The obtained solid was subjected to silica gel column chromatography (CH 2 Cl 2 :hexane=1:5, R f =0.30) to afford compound 24c-pre (7.73 g, 24.6 mmol) as a white solid in 50% yield.
[0346] Mp: 71.2-72.2°C.
[0347] 1 H NMR (300MHz, CDCl 3 , ppm) δ7.20-7.11 (m, 2H), 6.91-6.80ppm (m, 3H), 6.57 (td, 3 J(H,H)=8.7Hz, 4 J(H, H)=2.7Hz...
PUM
| Property | Measurement | Unit |
|---|---|---|
| carbon number | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com