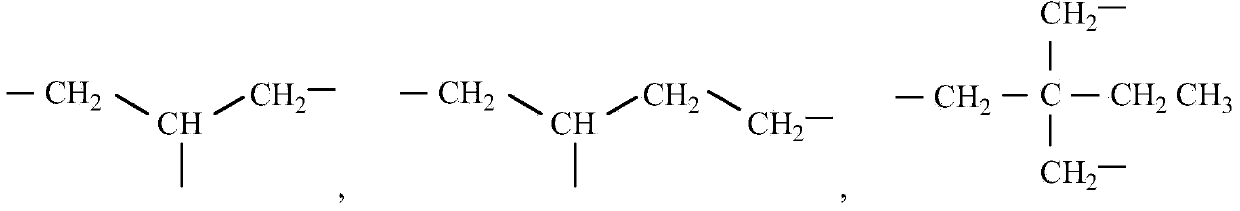
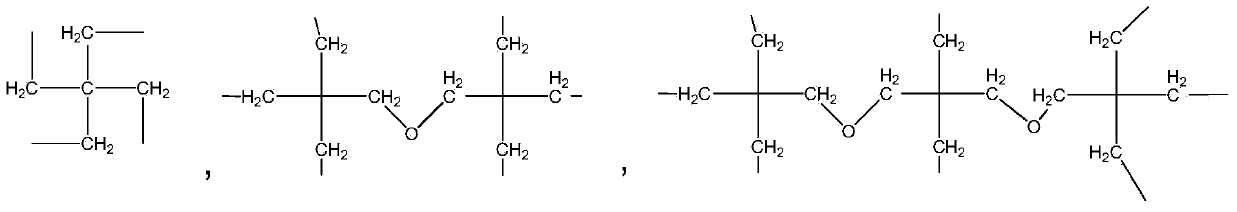
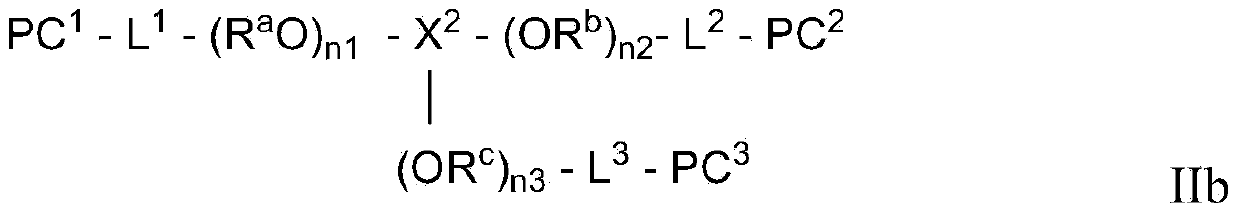Photochromic polymer
A photochromic and polymer technology, applied in the direction of color-changing fluorescent materials, optics, optical components, etc., can solve the problems of siloxane phase separation reduction, problems, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0190] step 1
[0191]
[0192] Poly(propylene oxide) (2.52 g, average molecular weight = 2,000, Aldrich), succinic anhydride (0.365 g) and p-toluenesulfonic acid (0.05 g) were mixed and heated at 125° C. for 30 minutes under nitrogen with stirring. The mixture was then dissolved in diethyl ether / hexane (4:1) and washed twice with 2M HCl and brine. Separate the organic layer with MgSO 4 Drying, filtration, and evaporation of the solvent in vacuo gave the product di(succinate) end-functionalized poly(propylene oxide) as a viscous oil. pass 1 H NMR (CDCl 3 ) analysis confirmed the structure as well as confirmed the completion of the reaction of the hydroxyl end groups of the starting polymer. The average molecular weight was determined to be 2,444 by integration of the analytical resonance peaks.
[0193] step 2
[0194]
[0195] The parent naphthopyran used in this step, 3,3-bis(4-methoxyphenyl)-13-hydroxy-13-methyl-indeno[2,1-f]naphtho[1,2 -b] pyran, synthesized u...
Embodiment 2
[0198] step 1
[0199]
[0200] Poly(propylene oxide) (5.02 g, average molecular weight = 1,000, Aldrich), succinic anhydride (2.50 g) and p-toluenesulfonic acid (0.13 g) were mixed and heated at 130° C. under nitrogen with stirring for 30 minutes and then in Heat at 80°C for an additional 1.5 hours. Poly(ethylene glycol) monomethyl ether (5.25 g, average molecular weight = 350, Aldrich) was added, and the mixture was heated at 100° C. for 30 minutes. The mixture was then dissolved in diethyl ether and washed three times with dilute HCl and finally with brine. Separate the organic layer with MgSO 4 Drying, filtering through a plug of silica gel, and evaporation of the solvent in vacuo gave the product di(succinate) end-functionalized poly(propylene oxide) as a viscous oil. pass 1 H NMR (CDCl 3 ) analysis confirmed the structure as well as confirmed the completion of the reaction of the hydroxyl end groups of the starting polymer. The average molecular weight was deter...
Embodiment 3
[0205] step 1
[0206]
[0207] Glycerol propoxylate (5.09 g, average molecular weight = 1,500), succinic anhydride (1.50 g) and triethylamine (2.10 mL) were added to dry dichloromethane (ca. 50 mL) under nitrogen and stirred overnight at ambient temperature . Poly(ethylene glycol) monomethyl ether (1.68 g, MW=350) was then added, the mixture was stirred for another 30 minutes, then the solvent was evaporated in vacuo. The residue was dissolved in diethyl ether and washed three times with dilute HCl and finally with brine. Separate the organic layer with MgSO 4 Drying, filtration and evaporation of the solvent gave the product as a viscous oil (5.11 g). pass 1 H NMR (CDCl 3 ) analysis confirmed the structure as tri(succinic acid / ester) end-functionalized glycerol propoxylate. The average molecular weight was determined to be 2,175 (n PPO =10.2 / arm).
[0208] step 2
[0209]
[0210] The tri(succinate) end-functionalized glycerol propoxylate from step 1 (0.26 g) ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com