Application of Eupalinolide K in preparing anticomplement drugs
An anti-complement and wild horse technology, applied in the direction of drug combination, pharmaceutical formula, medical preparations containing active ingredients, etc., to achieve the effect of shortening the separation cycle and improving the separation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: Preparation of Yemachai lactone K
[0025] Take 75 g of dried Ye Ma Zhui medicinal material, add 750 mL of ethanol and water mixed solution with a volume ratio of 80:20 for reflux extraction each time, extract twice, each time for 2 hours, filter the extract while it is hot, combine the filtrates, and recover ethanol under reduced pressure , to obtain 100 mL of aqueous suspension; extract 3 times with petroleum ether, each time with 200 mL of petroleum ether, evaporate the water layer to dryness, add 100 mL of methanol, dissolve it by ultrasonication for 15 minutes, reflux extraction for 1 hour, filter while hot, and recover the filtrate; The filtrate was left to settle at 4 °C for 2 hours, filtered, the filtrate was recovered to dryness under reduced pressure, and 4.936 g of sample was obtained by weighing; 4.936 g of sample was dissolved in 20 mL of a mixed solution of methanol and water with a volume ratio of 30:70. Phases were separated and purified, spec...
Embodiment 2
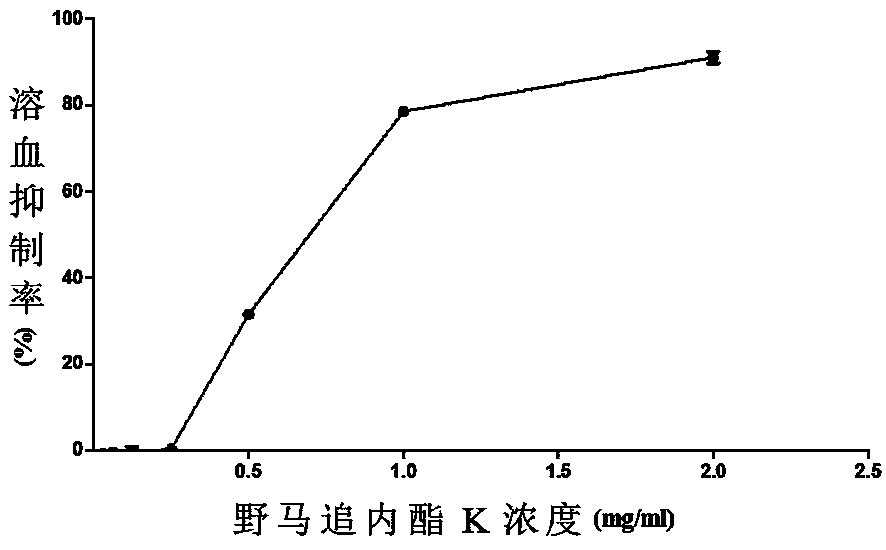
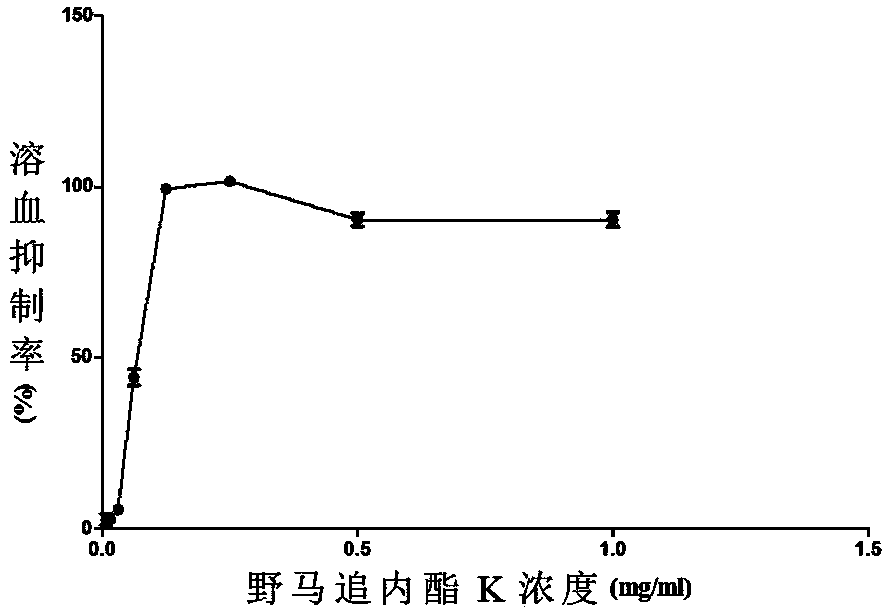
[0027] Example 2: Eupalinolide K Classical Pathway Complement Inhibition Experiment
[0028] 1. Experimental principle
[0029] Complement can hemolyze red blood cells sensitized by hemolysin. When the concentration of sensitized red blood cells is constant, the degree of hemolysis is proportional to the complement content. Therefore, after diluting the test product at different concentrations, mix it with complement, react with red blood cells, and measure the degree of hemolysis, so as to cause the minimum concentration of the test product required for 50% hemolysis (CH 50 ) to evaluate the anti-complement activity of the test article.
[0030] 2. Solution preparation
[0031] Barbitol buffer solution (BBS): take 2.875 g of barbital sodium, dissolve it in 250 mL of hot water, and ultrasonically vibrate to accelerate the dissolution, then add 42.5 g of NaCl, MgCl 2 ·6H 2 O 0.84 g, CaCl 2 0.14 g, 1.0 g of barbiturate, supplemented with triple distilled water to 1000 ml t...
Embodiment 3
[0044] Example 3: Eupalinolide K alternative pathway complement inhibition experiment
[0045] 1. Experimental principle
[0046] The Ca in the serum to be tested was chelated with ethylene glycol diethyl ether diamine tetraacetic acid (EGTA) 2+ , block the role of C1, block the classic activation pathway of complement, add rabbit red blood cells that can activate factor B, lead to activation of the alternative pathway of complement, rabbit red blood cells are damaged and hemolysis occurs. The relationship between the degree of hemolysis and the anti-complement activity of the test article is similar to the classic route, so the minimum concentration of the test article required to cause 50% hemolysis (AP 50 ) to evaluate the anti-complement activity of the test article.
[0047] 2. Solution preparation
[0048] 0.1M EGTA: Dissolve 3.5g of NaOH in 85mL of distilled water, add 19g of EGTA, dissolve, add distilled water to 500mL.
[0049] AP Diluent: 0.1 M EGTA 80 mL, Barbit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com