Tissue-factor-based tumor peptide vaccine as well as preparation method and application thereof
A tumor polypeptide vaccine and tissue factor technology, which is applied in the field of tumor polypeptide vaccine preparation, can solve the problems of complex protein structure, difficult process, allergic reaction, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
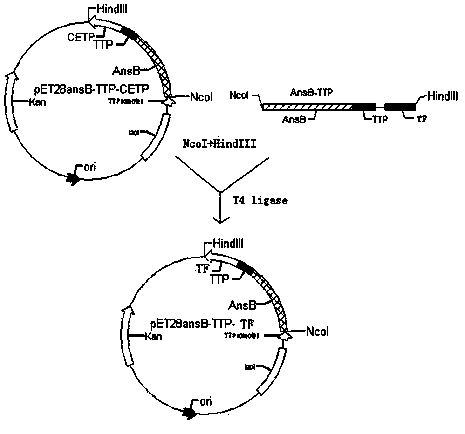
[0025] Embodiment 1: vector construction
[0026] 1.1 Cloning of AnsB-TTP-TF gene
[0027] The AnsB-TTP sequence comes from a plasmid vector already available in our laboratory. The AnsB-TTP-TF sequence can be obtained by adding the TF gene in two steps after the AnsB-TTP sequence by using the method of adding end PCR:
[0028] Design common upstream primers as:
[0029] A1: 5'-TAA TAC GAC TCA CTA TA - 3' (SEQ ID NO: 3)
[0030] Downstream primers are:
[0031] TF-down
[0032] The first step downstream primer TF-1: 5'- T GTC AGT AGT GTA GAA GCA TTT GGA TTT CCA GTC ACC GGT ACC AGA AGA GAT TAC AGA C-3' (SEQ ID NO: 4)
[0033] The second downstream primer TF-2: 5'- AAA AAGC TTA ACC GGT TTC GTC AGT CAA GTC GTA TTC AGT GTC AGT AGT GTA GAA GCA T-3' (SEQ ID NO: 5)
[0034] Upstream primers downstream contain restriction endonucleases Nco I site, the second downstream primer contains a restriction endonuclease Hind III site.
[0035] The upstream and downstream primers...
Embodiment 2
[0084] Embodiment two: transformation and screening
[0085] 2.1 Competent transformation
[0086] Take the above 5 μl connection solution to transform competent Escherichia coli BL21 (DE3) (for the preparation method, refer to the "Molecular Cloning Experiment Guide, Third Edition"), and the positive bacteria in the preliminary screening of kanamycin are then screened by enzyme activity assay, and the positive clones Extract the plasmid as a template, use the T7 promoter as the upstream primer, and use TF-2 as the downstream primer to express the vector pET28ansB-TTP-TF. The PCR conditions are the same as in Example 1.1; the clones that are positive by both screening methods are finally identified by DNA sequence assay to confirm the correct insertion of the AnsB-TTP gene.
[0087] Qualitative detection of asparaginase activity
[0088] The method for qualitative detection of asparaginase enzyme activity is the Naphthalene method: (boric acid buffer: H 2 BO 3 0.682...
Embodiment 3
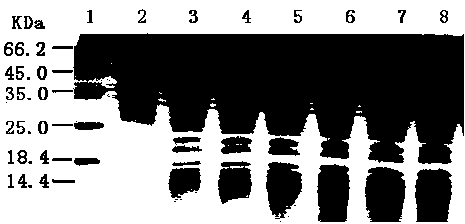
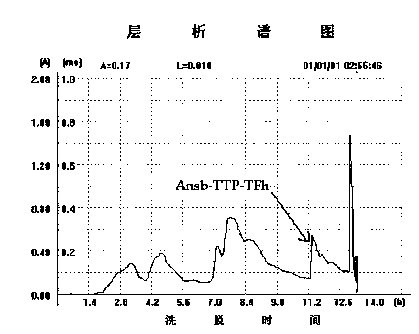
[0090] Example 3: Expression and purification of fusion protein
[0091] 3.1 Engineering bacteria amplification of pET28ansB-TTP-TF
[0092] The positive engineering bacteria obtained after screening were activated respectively, and a ring of bacteria was taken, cultured with shaking at 30°C overnight, inoculated into fresh liquid LB medium containing kanamycin at 1% inoculum size, and cultured at 37°C. Cultivate for 3.5 hours after transfer, add 1% culture volume of 0.5 M lactose solution to induce, so that the final concentration of lactose in the culture solution reaches 5 mM, continue to culture at 28°C for 4 hours, centrifuge at 5000 rpm for 10 minutes, and collect the bacteria.
[0093] Lysis of bacteria
[0094] Lysis buffer: 10 mM phosphate buffer (PB, pH8.0), 1 mM EDTA. Add 5 ml of lysis buffer per gram of bacteria, add 80 μl of lysozyme (10 mg / ml) and 20 μl of DNAase I (1 mg / ml), and lyse at 37°C for 1 h. 4 0 C, 10000 rpm, 15 min, the supernatant and the pr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com