Method for determining residual quantity of sulfur dioxide in Chinese herbal medicines
A technology of sulfur dioxide and Chinese medicinal materials, which is applied in the field of determination of residual sulfur dioxide in Chinese medicinal materials, and achieves the effects of low detection cost, safe operation and simple reaction method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1 The present invention establishes the rapid detection method of sulfur dioxide in Chinese medicinal materials
[0042] (1) Take 1g of the fine powder of the Chinese herbal medicine to be tested, add 25mL of 0.5% w / v sodium hydroxide solution, at 40°C, 200r min -1 Shake and extract for 30min, filter, take 1.0mL filtrate and dilute to 25mL to obtain the test solution;
[0043] (2) Take sulfite as the reference substance, and prepare concentrations of 1, 3, 7, 12, 15, 18×10 -3 mmol·L -1 The reference substance solution;
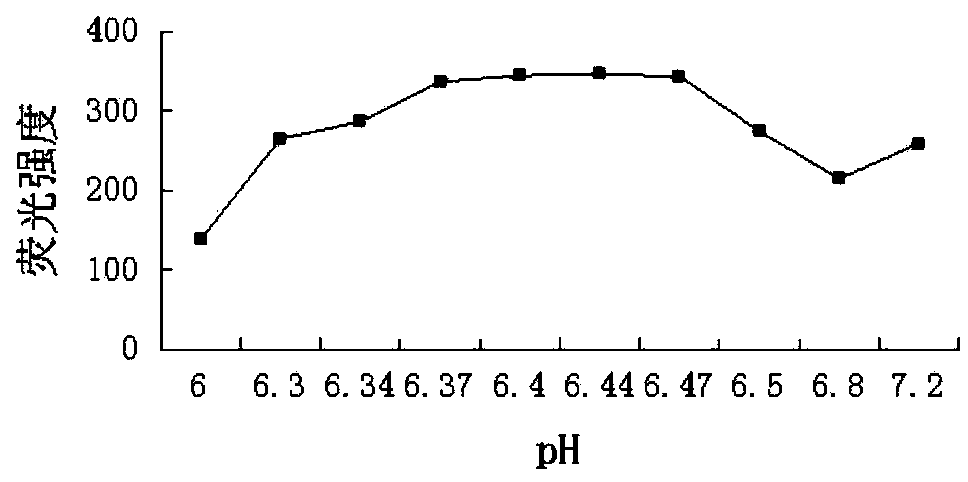
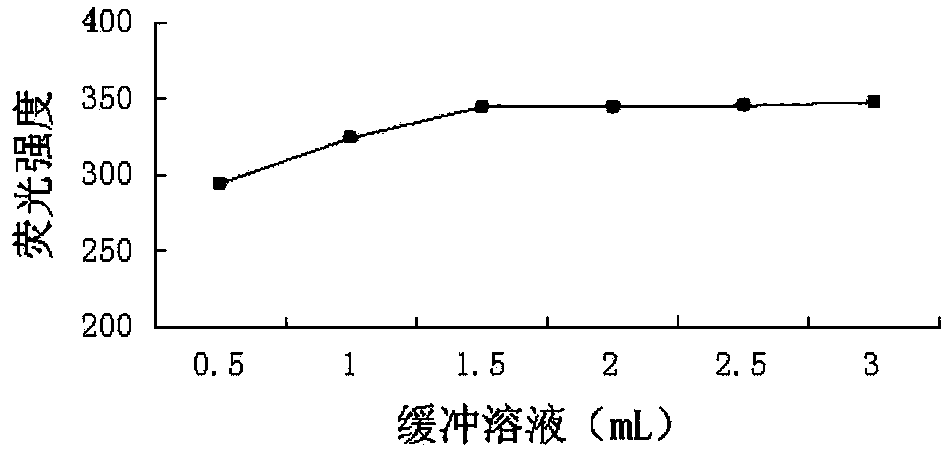
[0044] (3) Take an appropriate amount of the test solution and the reference solution in a 10ml volumetric flask, first add 2.0mL of Na with pH=6.44 2 HPO 4 -KH 2 PO 4 buffer solution, then add 2.5mL1mmoL·L -1 Phthalaldehyde solution and 1.5mL5mmoL·L -1 Ammonium acetate solution, mix well, dilute with water, keep the temperature in a water bath at 50°C for 5 minutes, take it out, immediately put it in ice water to cool, stop the react...
Embodiment 2
[0045] Embodiment 2 The optimization of detection method of the present invention
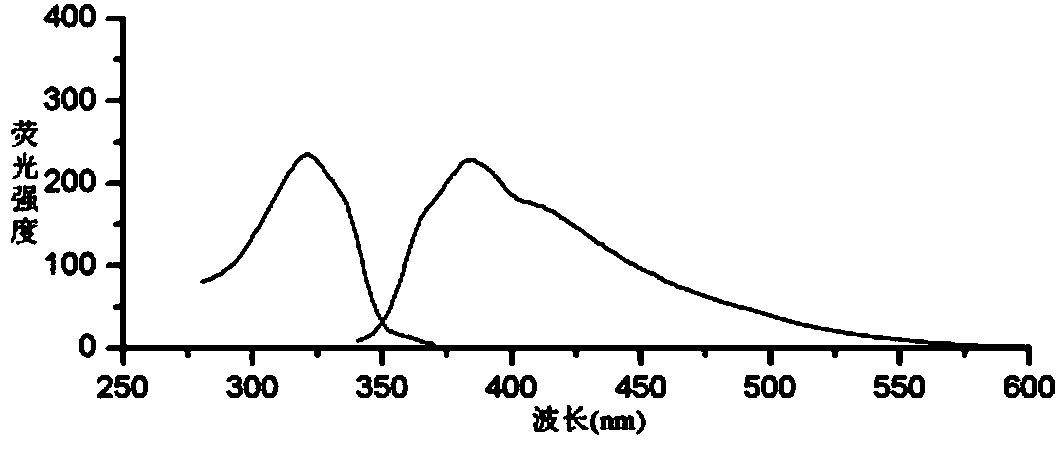
[0046] (1) Condition screening of fluorescence derivatization reaction method
[0047] 1 Experimental part
[0048] 1.1 Instrument
[0049] RF-5301 (PC) S fluorescence spectrophotometer (Shimadzu Corporation of Japan); electronic analytical balance BP211D (1 / 100,000, German Sartorius Company), electronic analytical balance BP121S (1 / 10,000, German Sartorius Company); pHS- 3C acidity meter (Shanghai Leici Instrument Factory); WHY-2 water bath constant temperature oscillator (Jincheng Guosheng Experimental Instrument Factory, Jintan City, Jiangsu); constant temperature water bath W201B (Beijing Guohua Medical Instrument Factory).
[0050] 1.2 Reagents
[0051]Sodium sulfite (Chengdu Kelong Chemical Reagent Factory, batch number: 20110906, purity ≥ 97%); o-phthalaldehyde (Japan TCI company, batch number: 120324, purity ≥ 98%); other reagents were analytically pure, and the experimental water wa...
Embodiment 3
[0088] Embodiment 3 methodological investigation
[0089] 1 Preparation of the test solution
[0090] Take about 1 g of yam fine powder, weigh it accurately, add 25 mL of 0.5% sodium hydroxide solution accurately, shake well, shake and extract (200r min -1 , 40°C) for 30min, filter, and dilute 1.0mL to 25mL.
[0091] 2 linear relationship test
[0092]Precisely draw 0.1, 0.3, 0.7, 1.2, 1.5 and 1.8mL of sodium sulfite stock solution and dilute to 100mL with water, and then pipette 1.0mL of the above-mentioned dilutions of different concentrations, and measure the relative fluorescence intensity of the product according to the experimental method. Taking the fluorescence intensity as the ordinate, Na 2 SO 3 The addition amount of the reference substance (nmoL) is the abscissa, and the standard curve is drawn, and the regression equation is: Y=46.247X-15.007, r 2 =0.9998, indicating that Na 2 SO 3 The linear relationship is good within the range of 0.9997-17.99nmoL.
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com