Preparation process of 2-naphthylamine-3,6,8-trisulfonic acid
A preparation process, amino technology, applied in the direction of sulfonic acid preparation, organic chemistry, etc., can solve the problems of reducing yield, increasing operation steps, etc., and achieve the effects of reducing pressure, high safety factor, and reducing loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
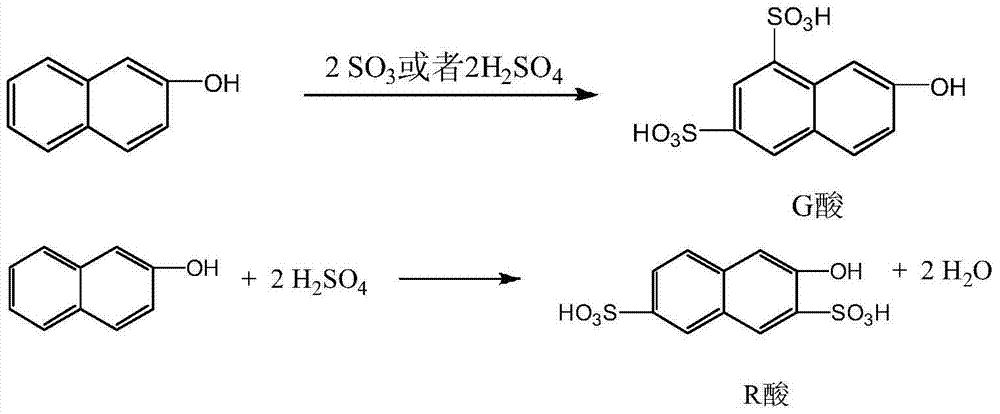
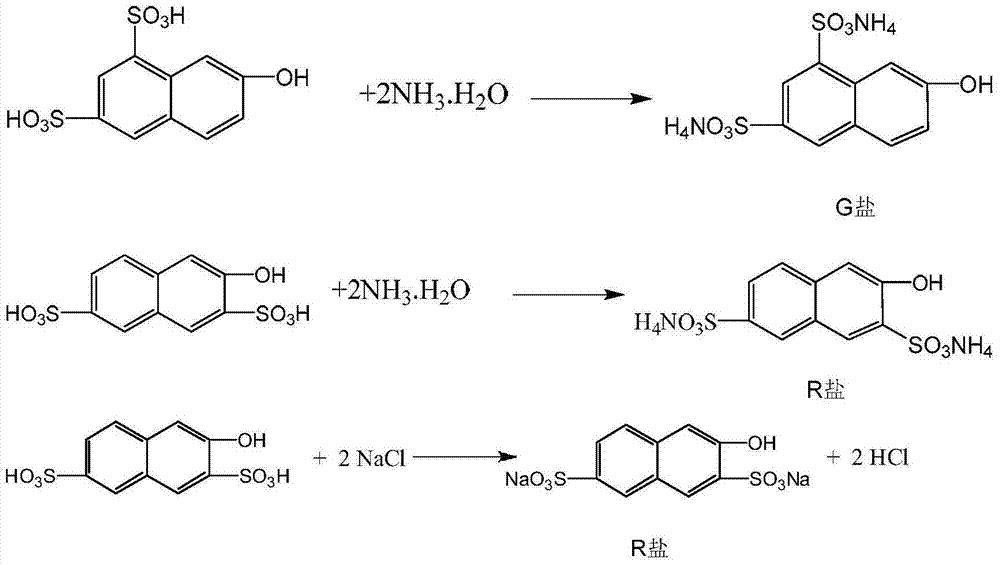
[0036] In a four-necked flask with stirring, add 200 grams of 100% sulfuric acid, add 150 grams of 2-naphthol in batches under stirring and cooling, control the feeding temperature at 40-60 ° C, then add 175 grams of 105% sulfuric acid, after the addition is complete Stir the reaction for 5 to 8 hours, and keep warm at 80°C for 7 hours to obtain the sulfonated compound; add the sulfonated compound to a solution of 221 grams of 20% ammonia water and 49 grams of sodium chloride, and keep warm at 85°C for 2 hours after the addition is completed. Hour, cooling, filters, obtains G salt and R salt mixture 320 grams, wherein G salt content 66.1%, R salt content 15.3%.
[0037] Dissolve 320 grams of the above G salt and R salt mixture in 165 grams of 20% ammonia water and 25 grams of catalyst (the molar ratio of ammonium bisulfate and sulfurous acid is 1:1), add it to the autoclave, raise the temperature to 130-140 ° C, and the pressure is 0.8-0.9MPa, maintain for 9-10 hours, after th...
Embodiment 2
[0042] In a 5000L glass-lined reactor, add 2000KG100% sulfuric acid, add 1500KG2-naphthol in batches under stirring and cooling, control the feeding temperature at 40-50°C, then add 1750KG105% sulfuric acid, stir and react for 5-8 hours after the addition is complete, After the reaction at 80°C for 7 hours, the sulfonate was added to a solution of 2200KG 20% ammonia water and 490KG sodium chloride, and after the addition was completed, it was kept at 85°C for 2 hours, cooled to 30°C, and filtered to obtain a mixture of G salt and R salt 3310KG, of which the G salt content is 65.3%, and the R salt content is 15.2%.
[0043] Dissolve 3310KG of the above G salt and R salt mixture in 1690KG of 20% ammonia water and 31KG of catalyst (the molar ratio of ammonium bisulfate and sulfurous acid is 1:1), put it into the autoclave, raise the temperature to 130-140°C, and the pressure is 0.8-0.9MPa , maintained for 8 to 12 hours, cooled down and diluted into water after the reaction, added...
Embodiment 3
[0047] In a four-necked flask with stirring, add 200 grams of 100% sulfuric acid, add 150 grams of 2-naphthol in batches under stirring and cooling, control the feeding temperature at 40-60 ° C, then add 175 grams of 105% sulfuric acid, after the addition is complete Stir the reaction for 5 hours, and then keep it warm at 80°C for 7 hours. After the reaction, the sulfonated compound is obtained; the sulfonated compound is added to the solution of 200 grams of 20% ammonia water and 55 grams of sodium chloride, and after the addition is completed, it is kept at 85° C. for 2 hours. Hour, cooled to 30 ℃ and left standstill for 2 hours, filtered to obtain 312 grams of G salt and R salt mixture, wherein the G salt content was 65.9%, and the R salt content was 15.7%.
[0048] Dissolve 312 grams of the above G salt and R salt mixture in 165 grams of 20% ammonia water and 25 grams of catalyst (the molar ratio of ammonium bisulfate and sulfurous acid is 1:1.2), add it to the autoclave, rai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com