Method for synthesizing silodosin
A technology of silodosin and a synthesis method, which is applied in the field of preparation of silodosin, a drug for the treatment of benign prostatic hyperplasia, can solve the problems of difficult reaction operation, unfavorable industrial production, complicated preparation process, etc., and achieve simplified labor intensity and easy The effect of industrial production and controllable optical purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
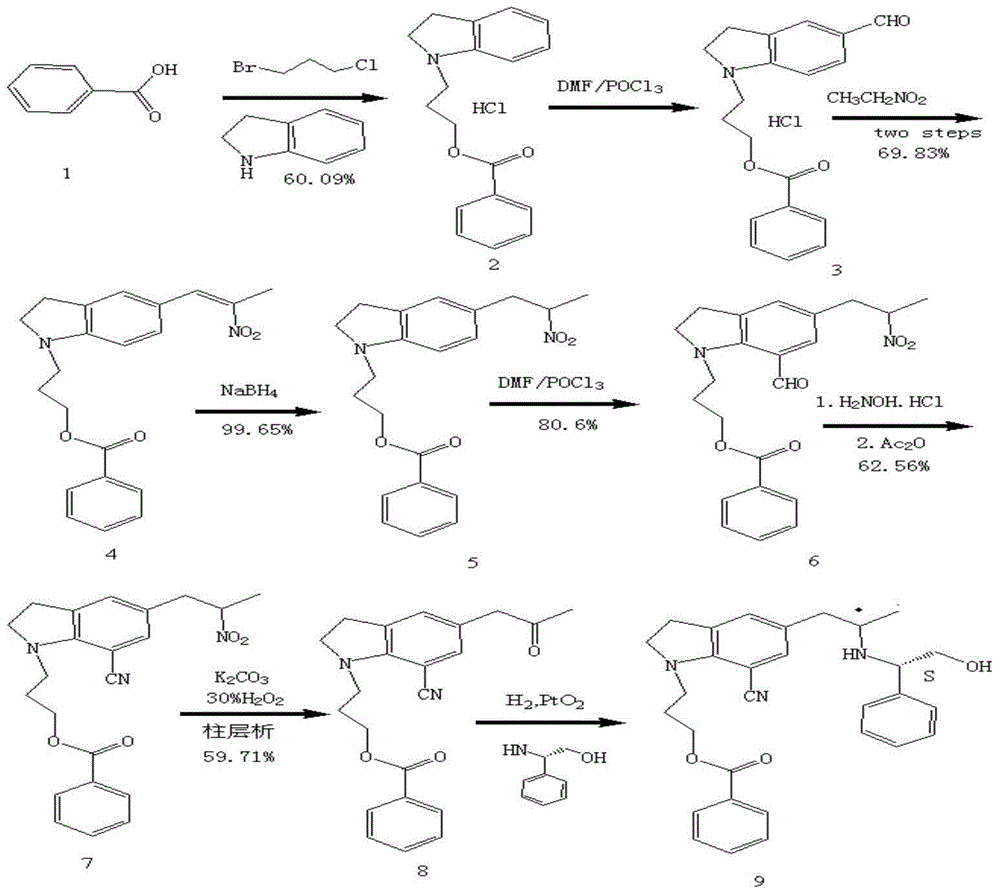
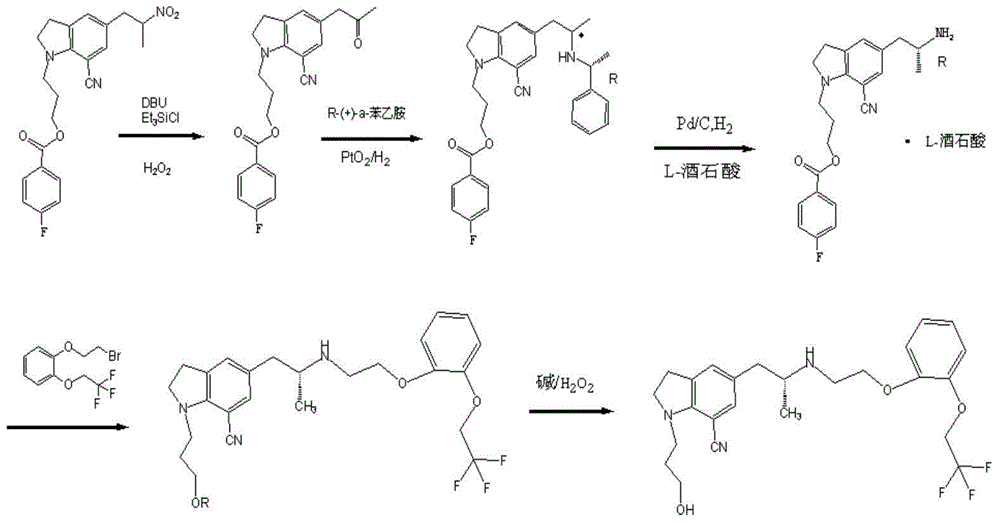
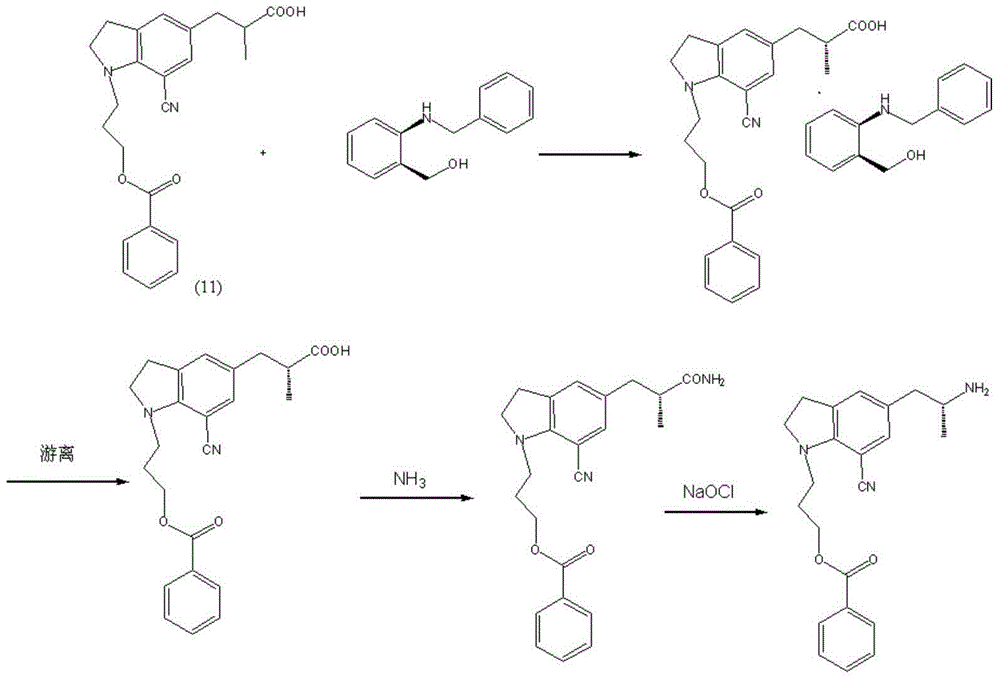
[0043] Example 1, a synthetic method of silodosin, the steps are as follows: 7-cyanoindoline is used as the starting material, and a benzoyloxypropyl group is introduced into the 1 position of indoline to synthesize 1-(benzoyloxypropyl)-7-cyanoindoline compound (I), and then with 2R-2-[(phenylmethyl)amino]-propionyl chloride compound (II) by Friedel-Crafts Synthesis of key chiral intermediate 5-[(2R)-2-(benzylamino)-1-propanone]-1-[3-(benzoyloxy)propyl]-7-cyano through acylation reaction The indoline compound (III) was reduced by triethylsilane to obtain 5-[(2R)-2-(benzylamino)propyl]-1-[3-(benzoyloxy)propyl]- 7-cyanoindoline compound (IV), and then through catalytic hydrogenation to obtain (R)-1-[1-(3-benzoyloxypropyl)-5-(2-aminopropyl)7- A cyano]indoline compound (V) undergoes a condensation reaction with a 2-(2,2,2,-trifluoroethoxy) phenoxyethyl methanesulfonate compound (VI) under basic conditions to obtain 1 -(3-(4-fluorobenzoyl)hydroxypropyl)-5-((2R)-2-(2-(2-(2,2,2-tri...
Embodiment 2
[0044] Example 2, in the method described in Example 1: 7-cyanoindoline is used as raw material and 3-chloropropyl benzoate to react to obtain the compound of formula (I), and the specific steps are as follows: 1.0 parts by weight 3-chloropropyl benzoate and 1.6 parts by weight of 7-cyanoindoline were reacted in 5-10 times the amount of polar solvent at 80-100°C for 10-15 hours, and finally hydrochloric acid was added to form a salt to obtain Compound (I) hydrochloride; the polar solvent is selected from DMF or DMSO, and an acid reducing agent is added to DMF or DMSO.
Embodiment 3
[0045] Embodiment 3, in the method described in embodiment 2: the acid reducing agent that adds in the DMF or DMSO is the triethylamine or pyridine of 0.84 weight part.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com