Reaction-type halogen amine antibacterial agent, and synthetic method and application thereof
An antibacterial agent and reactive technology, which is applied in the synthesis field of reactive haloamine antibacterial agents, can solve the problems affecting the application and antibacterial performance, the bactericidal efficiency needs to be improved, and the poor water solubility of antibacterial precursors, etc., to achieve superior antibacterial Sterilization function, short reaction time, low price effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 3
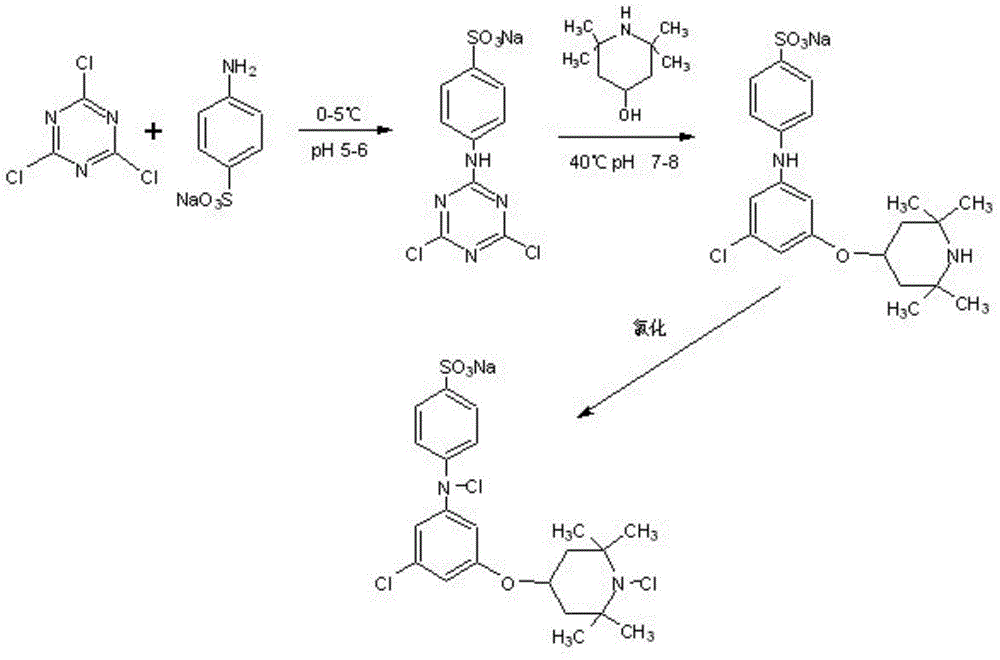
[0034] Haloamine antibacterial agent precursor 2-fluoro-4-(4-sulfoanilino)-6-(2,2,6,6-tetramethylpiperidin-4-oxyl)-1,3, Preparation of 5-triazine
[0035] Weigh 5.53g of cyanuric fluoride, dissolve it in 40mL of carbon tetrachloride, weigh 5.20g of p-aminobenzenesulfonic acid and 1.59g of sodium carbonate, dissolve it in 30mL of water, and mix the two in a 250mL three-necked flask and placed in an ice bath, reacted for 4 hours at a pH value of 5.0 to 6.0, then weighed 4.72g of 2,2,6,6-tetramethyl-4-piperidinol and dissolved it at 60°C for reaction After 4 hours, filter and wash with acetone and ice water, then dry and store.
[0036] Tests have shown that under alkaline conditions, the solubility of the antibacterial agent precursor prepared in Examples 1 to 3 in water can reach 10%.
[0037] 2. Application examples of precursors of reactive halamine antibacterial agents
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com