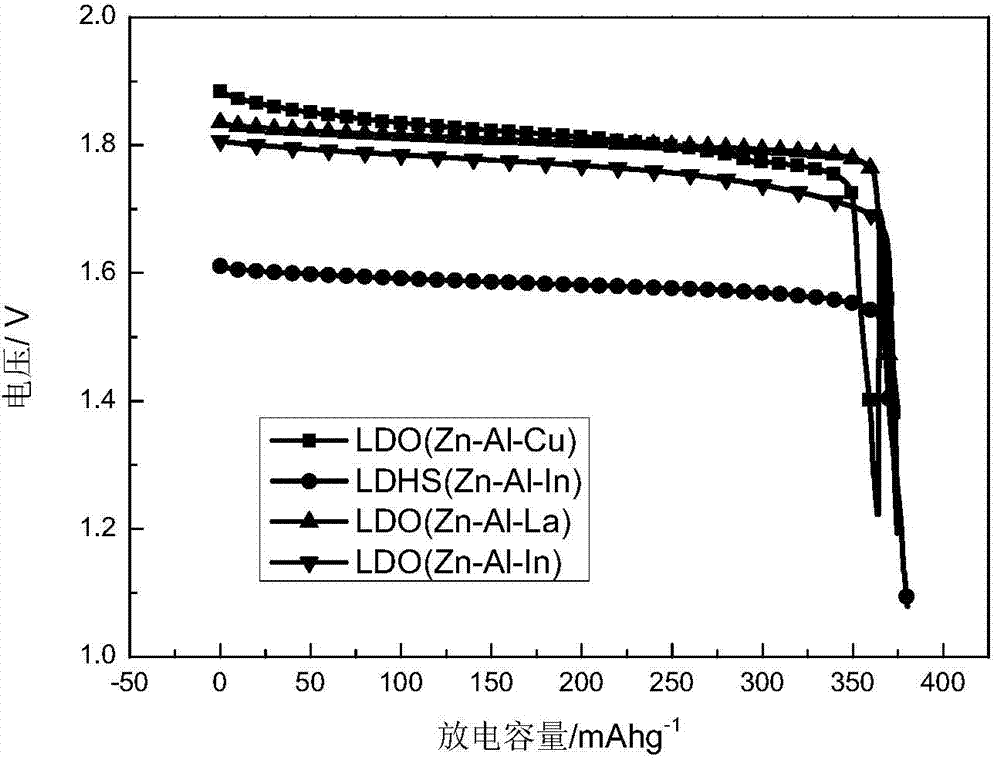
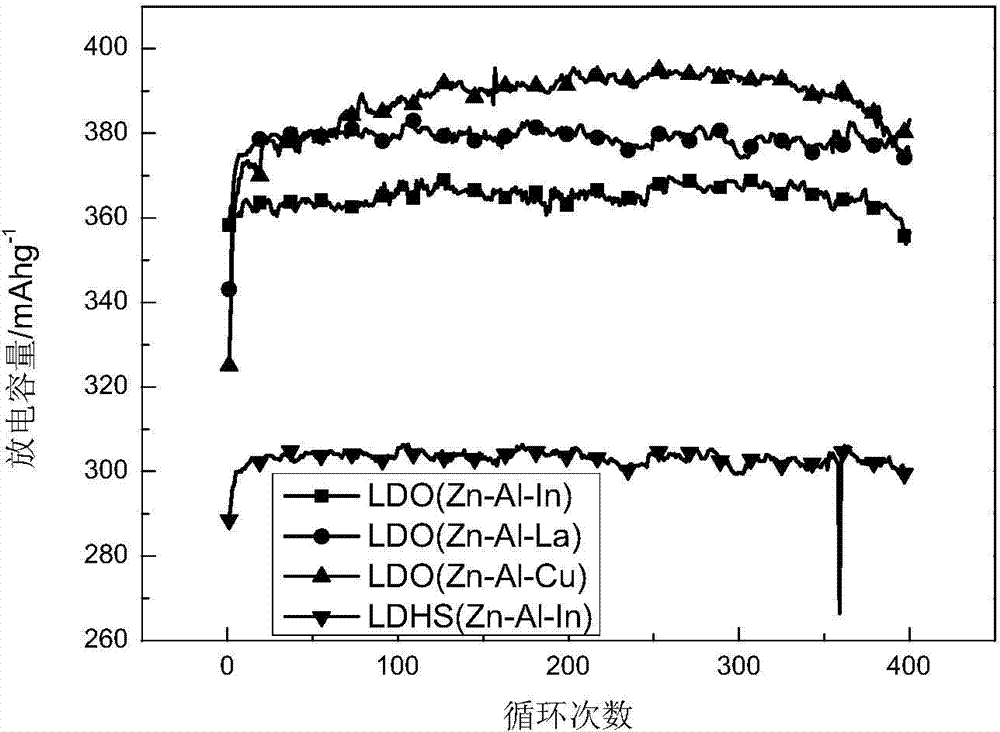
Application of zinc-based ternary layered composite oxide to zinc-nickel battery electrode material
A composite oxide, zinc-based ternary layer technology, applied in battery electrodes, alkaline storage battery electrodes, circuits, etc., can solve problems such as reducing the capacity of zinc oxide, achieve improved electrochemical performance, improved dendrites, and increased utilization Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The analytically pure zinc nitrate of 8.95 parts by weight is dissolved respectively in the deionized water of 100 parts by weight, the analytically pure aluminum nitrate of 2.25 parts by weight and the analytically pure indium nitrate of 1.24 parts by weight are dissolved in the deionized water of 100 parts by weight, Mix the above three solutions evenly to form a salt solution. 3.4 parts by weight of sodium hydroxide and 2.10 parts by weight of sodium carbonate were dissolved in 50 parts by weight of deionized water to form an alkaline solution. Under strong mechanical stirring, slowly add the salt solution and alkali solution dropwise into 50 parts by weight of deionized water, control the pH of the solution to 10.0, continue to stir for 80 minutes, age for 20 hours, and finally filter, wash and remove the precipitate. Dry at 60°C for 4 hours to obtain a carbonate-type zinc aluminum indium hydrotalcite sample. The hydrotalcite is placed in a muffle furnace and calci...
Embodiment 2
[0031]The analytically pure zinc nitrate of 8.95 parts by weight is dissolved in 100 parts by weight of deionized water, the analytically pure aluminum nitrate of 2.25 parts by weight and the analytically pure lanthanum nitrate of 1.26 parts by weight are dissolved in 100 parts by weight of deionized water, Mix the above three solutions evenly to form a salt solution. 5.60 parts by weight of potassium hydroxide and 2.76 parts by weight of potassium carbonate were dissolved in 50 parts by weight of deionized water to form an alkaline solution. Under strong mechanical stirring, slowly add salt solution and alkali solution dropwise into 50 parts by weight of deionized water, control the pH of the solution to 10.0, continue to stir for 100 minutes, age for 20 hours, and finally filter, wash and remove the precipitate. Dry at 60°C for 4 hours to obtain a carbonate-type zinc-aluminum-lanthanum hydrotalcite sample. The hydrotalcite is placed in a muffle furnace and calcined at a hig...
Embodiment 3
[0033] The analytically pure zinc nitrate of 8.95 weight parts is dissolved in the deionized water of 100 weight parts, the analytically pure aluminum nitrate of 2.25 weight parts and the analytically pure copper nitrate of 1.26 weight parts are dissolved in the deionized water of 100 weight parts, Mix the above three solutions evenly to form a salt solution. 6.72 parts by weight of potassium hydroxide and 2.76 parts by weight of potassium carbonate were dissolved in 50 parts by weight of deionized water to form an alkaline solution. Under strong mechanical stirring, slowly add the alkali solution and the alkali solution dropwise into 50 parts by weight of deionized water, control the pH of the solution to 10.0, continue to stir for 120 minutes, age for 20 hours, and finally filter, wash, and remove the precipitate. Dry at 60°C for 4 hours to obtain a carbonate-type zinc-aluminum-copper hydrotalcite sample. The hydrotalcite is placed in a muffle furnace and calcined at a high...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com