Grid-structured carbon-coated lithium iron phosphate nanoparticles and preparation method and application thereof
A technology for carbon-coated lithium iron phosphate and nanoparticles, which is applied in the fields of nanomaterials and electrochemistry, can solve the problems of low conductivity of lithium ion batteries, slow diffusion rate of lithium ions, etc., achieves convenient and repeatable preparation methods, and promotes electron conduction. rate, the effect of reducing the polarization effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1 of the present invention provides a method for preparing grid-structured carbon-coated lithium iron phosphate nanoparticles, comprising the following steps:
[0026] Step S1, weigh 5g of solid lithium iron phosphate and put it into a beaker containing 100 mL of distilled water, and stir at 80° C. for 30 min until all the solids are dissolved to obtain a lithium iron phosphate solution;
[0027] Step S2, add 0.25g cetyltrimethylammonium bromide to the lithium iron phosphate solution, then immerse the beaker in cold water and cool to room temperature, then heat it in a water bath at 80°C for 10h, collect the precipitate, and wash with distilled water and ethanol Precipitate multiple times to obtain solid precipitate;
[0028] In step S3, the solid precipitate was dried at 120° C. for 20 h, and then dried at 100 mL / min N 2 In a flow rate atmosphere, the temperature of the calcining furnace was first increased from room temperature to 100 °C within 30 minutes,...
Embodiment 2
[0036] The difference between Example 2 and Example 1 is only that the addition amount of cetyltrimethylammonium bromide is 0.75 g; the rest are basically the same as Example 1.
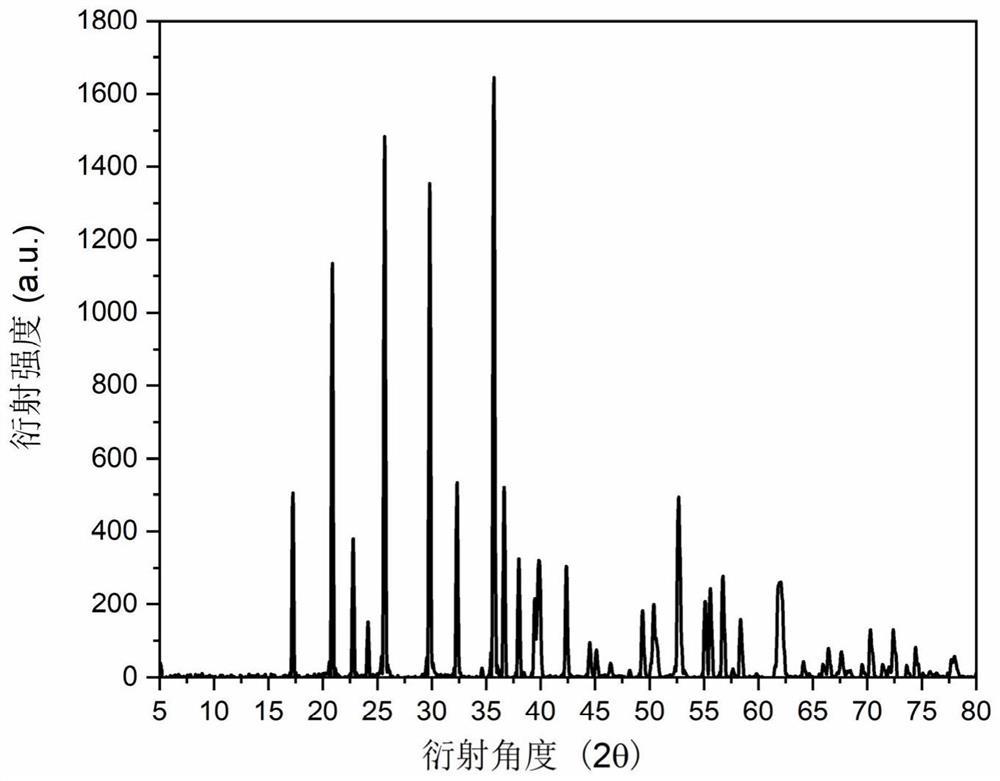
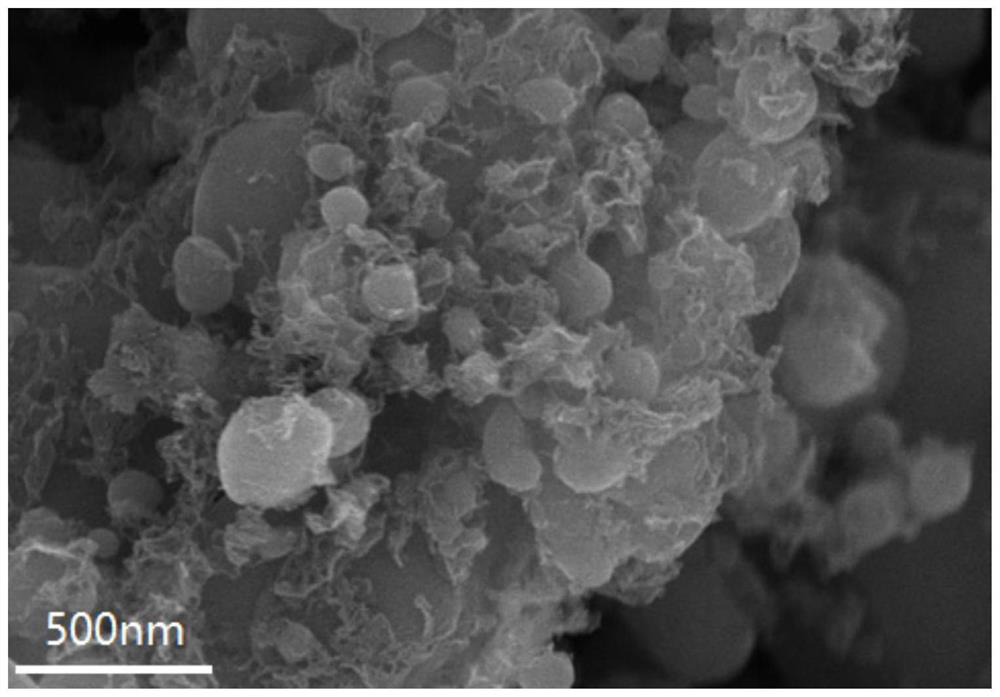
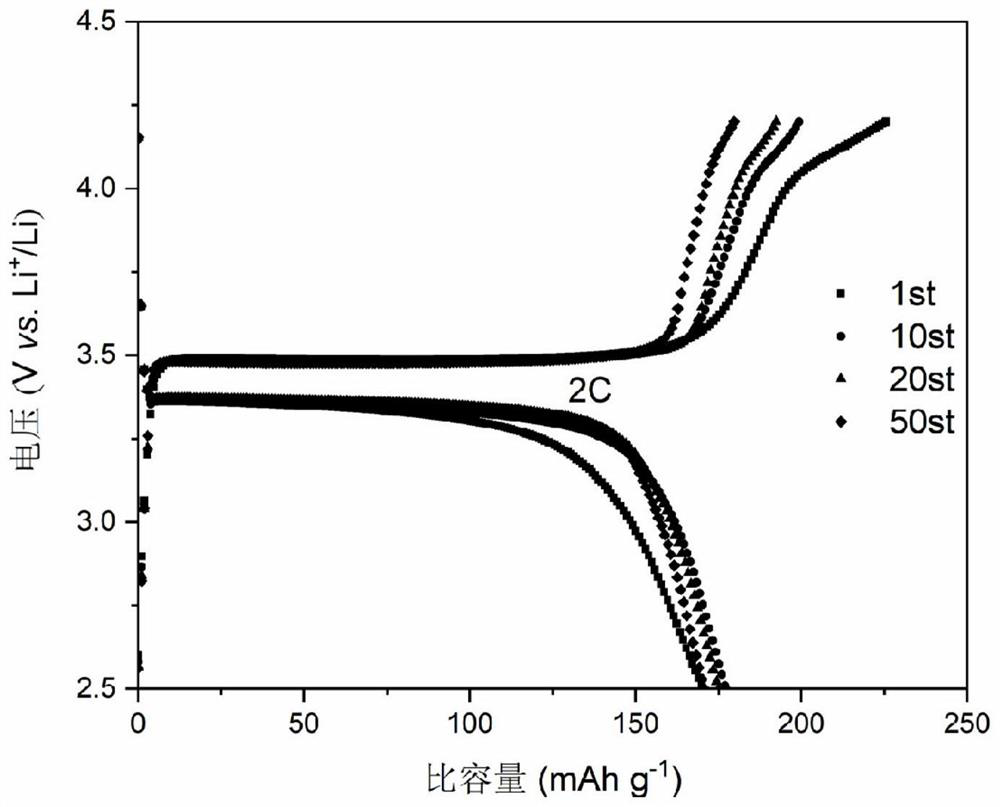
[0037] The carbon-coated lithium iron phosphate nanoparticles prepared in Example 2 were subjected to X-ray testing, scanning electron microscope testing, transmission electron microscopy, and electrochemical performance tests for coin cells. The X-ray testing showed that the prepared product carbon-coated lithium iron phosphate nanoparticles The particle morphology and size are uniform; the positive electrode material of carbon-coated lithium iron phosphate nanoparticles obtained in this example is assembled into a half-cell, and the lithium sheet is used as the negative electrode, and a button battery is used for testing. The constant current charge-discharge test shows that the electrode assembled by the carbon-coated lithium iron phosphate nanoparticle cathode material is at a current density of 2...
Embodiment 3
[0039] The only difference between Example 3 and Example 1 is that the calcination temperature is 800° C.; the rest are basically the same as Example 1.
[0040]The carbon-coated lithium iron phosphate nanoparticles prepared in Example 3 were subjected to X-ray testing, scanning electron microscope testing, transmission electron microscopy, and electrochemical performance tests for coin cells. The X-ray testing showed that the prepared product carbon-coated lithium iron phosphate nanoparticles The particle morphology and size are uniform; the positive electrode material of carbon-coated lithium iron phosphate nanoparticles obtained in this example is assembled into a half-cell, and the lithium sheet is used as the negative electrode, and a button battery is used for testing. The constant current charge-discharge test shows that the carbon-coated lithium iron phosphate nanoparticle cathode has a specific capacity of 154mAh g for the first discharge at a current density of 2C. -...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com