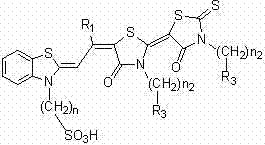
Preparation method for bis(rhodanine)merocyanine sensitizing dye with a benzothiazole skeloton
A technology of benzothiazole and sensitizing dyes, applied in organic dyes, chemical instruments and methods, methine/polymethine dyes, etc., to achieve the effects of low environmental pollution, high product purity, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
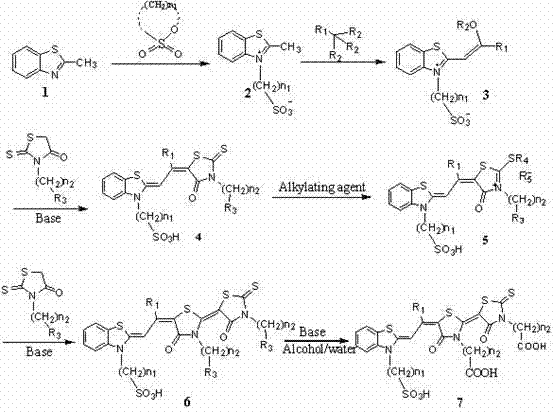
[0025] (1) Add 14.9 g (0.1 mol) 2-methylbenzothiazole, 18.3 g (0.15 mol) 1,3-propane sultone, 100 mL toluene into a three-necked flask, and heat to reflux for 15 h. Cool, filter, and dry to obtain 14.9 g of solids, with a yield of 85%. 1 H NMR (400 MHz, D 2 O-d 2 ), δ : 7.64~8.07 (m, 4H, Ar—H), 4.77~4.78,(t, J =8.0 Hz, 2H, N—CH 2 ), 3.07 (s, 3H, —CH 3 ), 3.00~3.02 (t, J =6.0 Hz, 2H, CH 2 —S), 2.24~2.31 (m, 2H, —CH 2 —); MS (ESI), m / z : 272, 294.
[0026] (2) Add 13.5 g (0.05 mol) of 2-methyl-3-(3 , -Propylsulfonate)benzothiazole, add 15 mL of cresol, raise the temperature to 120 °C, add 14.1 g (0.08 mol) triethyl orthopropionate dropwise, and continue the reaction for 2 h. After cooling, 100 mL of ethyl acetate was added to precipitate a solid, which was filtered and dried to obtain 13.1 g of a solid with a yield of 73.8%. m.p. above 300 ℃ (decomposition); 1 H NMR (400 MHz, CD 3 OD-d 4 ), δ : 7.63~8.15 (m, 4H, Ar—H), 6.68 (s, 1H, —CH=), 4.90~4.94,(t, J =8.0...
Embodiment 2
[0031] (1) Add 14.9 g (0.1 mol) 2-methylbenzothiazole, 20.4 g (0.15 mol) 1,4-butane sultone, and 100 mL chlorobenzene into a three-necked flask, and heat to reflux for 15 h. Cool, filter, and dry to obtain 23.1 g of solids, with a yield of 81%.
[0032] (2) Add 14.3 g (0.05 mol) of 2-methyl-3-(3 , -Butylsulfonate)benzothiazole, add 15 mL of cresol, raise the temperature to 120 °C, add 16.2 g (0.1 mol) triethyl orthoacetate dropwise, and continue the reaction for 2 h. After cooling, 100 mL of ethyl acetate was added to precipitate a solid, which was filtered and dried to obtain 16.5 g of a solid with a yield of 93%.
[0033] (3) Add 17.7 g (0.05 mol) of 2-(2-ethoxy-1-propenyl)-1-(3-sulfonic acid butyl) benzothiazole and 13.1 g (0.06 mol) For rhodanine methyl propionate, 20 mL of absolute ethanol was added, the temperature was raised to reflux, 0.085 g (0.01 mol) of piperidine was added dropwise, and the reaction was continued for 2 h. Cool, evaporate methanol to dryness, add...
Embodiment 3
[0038] (1) Add 14.9 g (0.1 mol) 2-methylbenzothiazole, 18.3 g (0.15 mol) 1,3-propane sultone, 100 mL toluene into a three-necked flask, and heat to reflux for 15 h. Cool, filter, and dry to obtain 14.9 g of solids, with a yield of 85%.
[0039] (2) Add 14.3 g (0.05 mol) of 2-methyl-3-(3 , -Propylsulfonate)benzothiazole, add 15 mL of cresol, raise the temperature to 120 °C, add 16.2 g (0.1 mol) trimethyl orthopropionate dropwise, and continue the reaction for 2 h. After cooling, 100 mL of ethyl acetate was added to precipitate a solid, which was filtered and dried to obtain 14.7 g of a solid with a yield of 90%.
[0040] (3) Add 16.5 g (0.05 mol) of 2-(2-methoxy-1-propenyl)-1-(3-sulfonic acid butyl) benzothiazole and 12.5 g (0.06 mol) Add 20 mL of absolute ethanol to rhodanine methyl acetate, heat up to reflux, add 0.075 g (0.01 mol) pyridine dropwise, and continue the reaction for 2 h. Cool, evaporate methanol to dryness, add 50 mL of acetone, and precipitate a solid. Filt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com