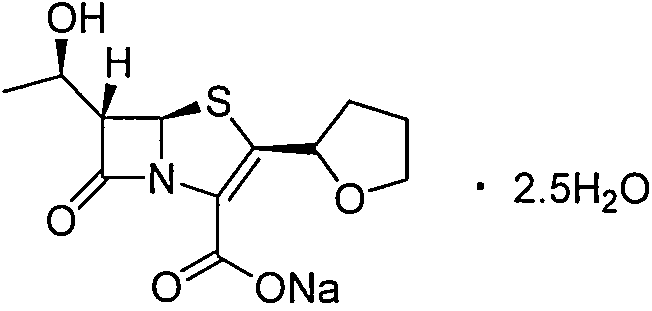
Purification method of faropenem sodium hydrate
A technology of faropenem sodium and ropenem sodium, which is applied in the field of drug hydrates, can solve problems such as easy decomposition, difficult recovery, and difficult recrystallization, and achieve the effect of increasing crystallization yield and reducing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~11
[0017] Step 1) In a dry 500mL three-neck flask, add the crude faropenem sodium hydrate (content 98.0%) and lower alcohol solvent, T 1 After stirring and dissolving at °C, 2.5 g of activated carbon was added, stirred at room temperature for 15 minutes, and filtered to obtain the filtrate.
[0018] Step 2) Add deionized water to the filtrate and control the temperature at T 2 The lower alcohol solvent was evaporated under reduced pressure at ℃ to obtain a residue.
[0019] Step 3) Add a poorly soluble ketone solvent to the residue and cool to T 3 ℃ and controlled to crystallize at this temperature, keep for t hours, filter, vacuum dry to obtain off-white crystals.
[0020] Table 1 Crystallization process parameters of Examples 1-11
[0021]
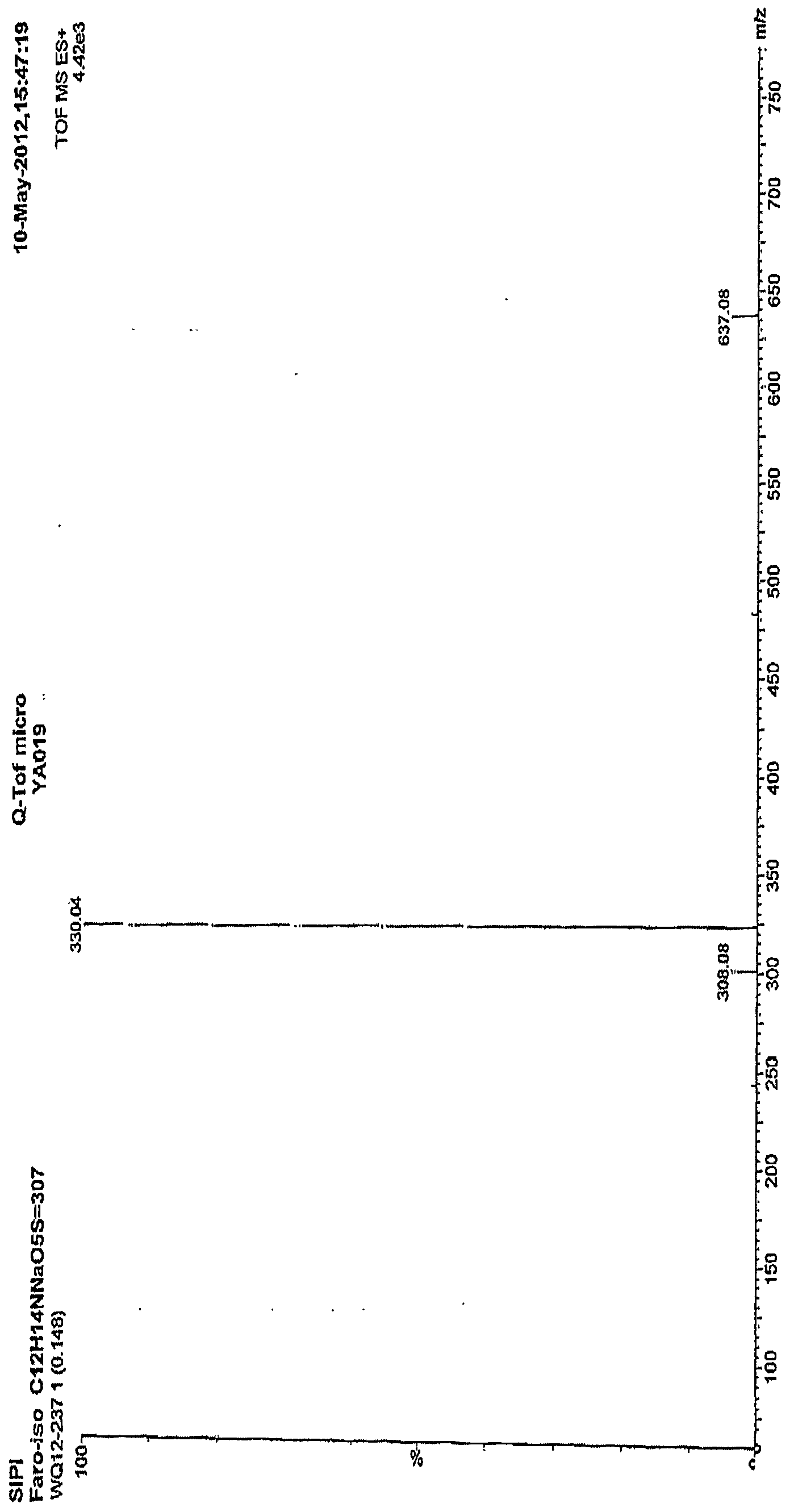
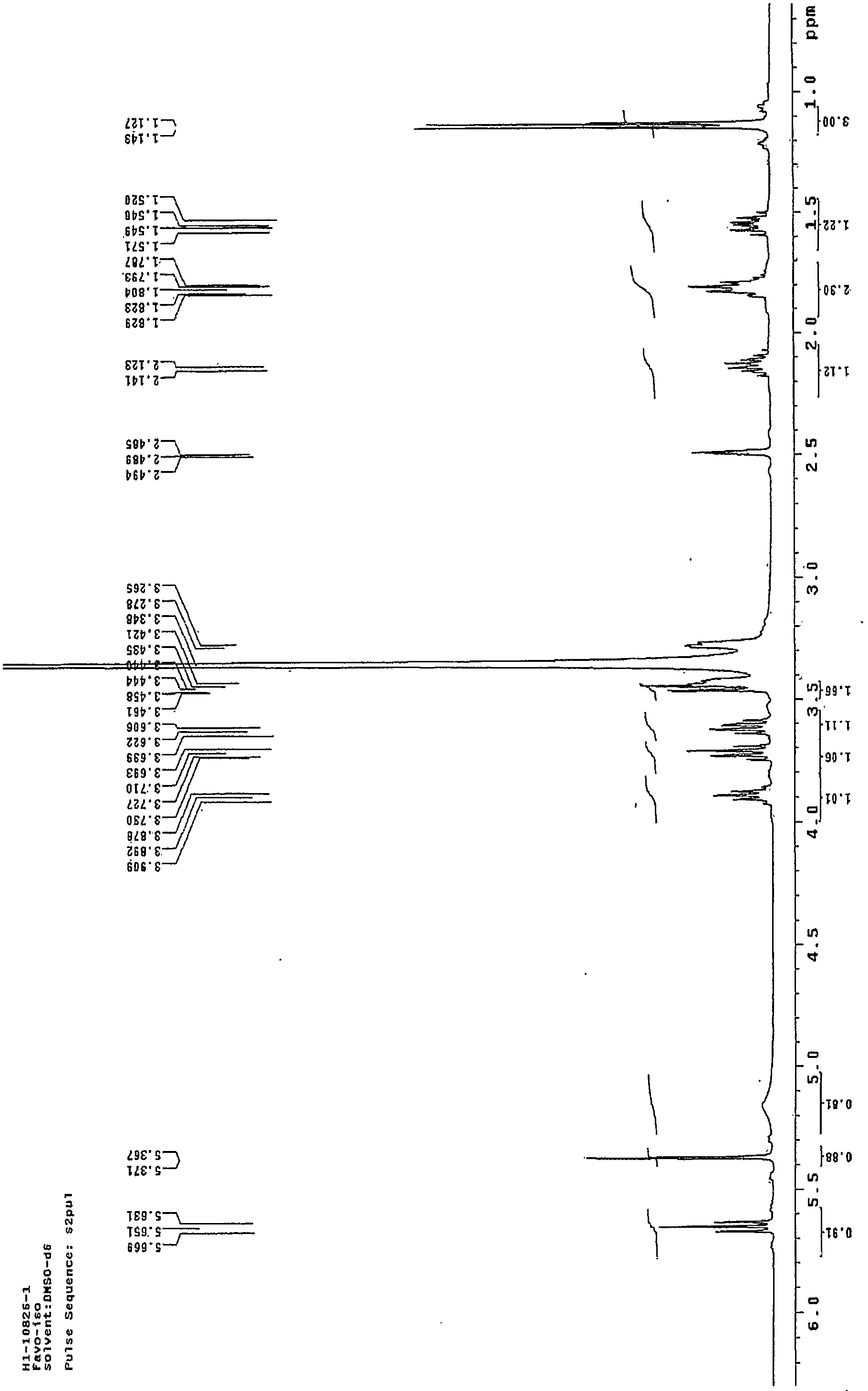
[0022] figure 1 Is the mass spectrum of the pure product of Faropenem Sodium Hydrate obtained in Example 1; figure 2 This is the NMR spectrum of the pure faropenem sodium hydrate obtained in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com