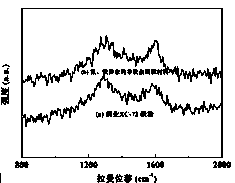
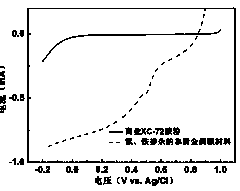
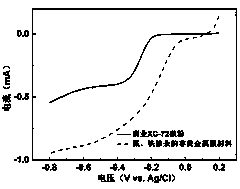
Iron and nitrogen doped non-noble metal catalyst as well as preparation method and application thereof
A non-precious metal and catalyst technology, applied in the field of non-precious metal catalysts and oxygen reduction catalytic reactions, can solve the problems of large-scale commercial production limitations, expensive prices, limited content, etc., and achieve the effect of aerobic reduction performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Weigh 1000g of sucrose, 250g of 6-benzyl adenine, FeCl 3 50g, mixed thoroughly, added to 20ml of concentrated sulfuric acid, oxidized and carbonized at room temperature for 14 days, washed, dried, and ball milled with a ball mill; finally, in a tube furnace, under nitrogen protection, graphitized at 600°C for 2 hours to obtain chlorinated Iron-activated nitrogen-containing purine cathode catalyst.
[0019] Through the oxygen reduction test, it was found that on the nitrogen-containing purine cathode catalyst electrode activated by ferric chloride under acidic conditions, the onset potential and half-wave potential of the oxygen reduction reaction were positively shifted by 487mV and 293mV respectively; The onset potential and half-wave potential of the reaction shifted positively by 220 mV and 56 mV, respectively.
Embodiment 2
[0021] Weigh 1250g of sucrose, 250g of 6-benzyl adenine, FeCl 3 50g, mixed thoroughly, added to 20ml of concentrated sulfuric acid, oxidized and carbonized at room temperature for 14 days, washed, dried, and ball milled with a ball mill; finally, in a tube furnace, under nitrogen protection, graphitized at 700°C for 1 hour to obtain chlorinated Iron-activated nitrogen-containing purine cathode catalyst.
[0022] Through the oxygen reduction test, it was found that on the nitrogen-containing purine cathode catalyst electrode activated by ferric chloride under acidic conditions, the onset potential and half-wave potential of the oxygen reduction reaction were positively shifted by 466mV and 287mV respectively; The onset potential and half-wave potential of the reaction shifted positively by 215 mV and 52 mV, respectively.
Embodiment 3
[0024] Weigh 1000g of sucrose, 250g of 6-benzyl adenine, FeCl 3 50g, mixed thoroughly, added to 20ml of concentrated sulfuric acid, oxidized and carbonized at room temperature for 14 days, washed, dried, and ball milled with a ball mill; finally, in a tube furnace, under nitrogen protection, graphitized at 800°C for 2 hours to obtain chlorinated Iron-activated nitrogen-containing purine cathode catalyst.
[0025] Through the oxygen reduction test, it was found that on the nitrogen-containing purine cathode catalyst electrode activated by ferric chloride under acidic conditions, the onset potential and half-wave potential of the oxygen reduction reaction were positively shifted by 550mV and 388mV respectively; The onset potential and half-wave potential of the reaction shifted positively by 242 mV and 71 mV, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com