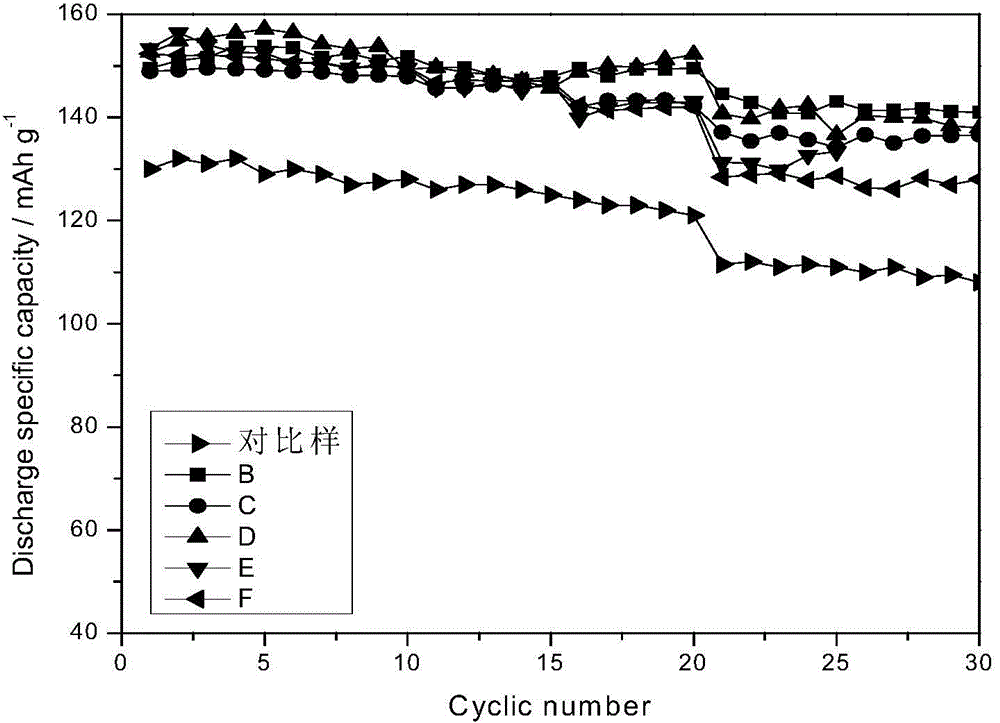
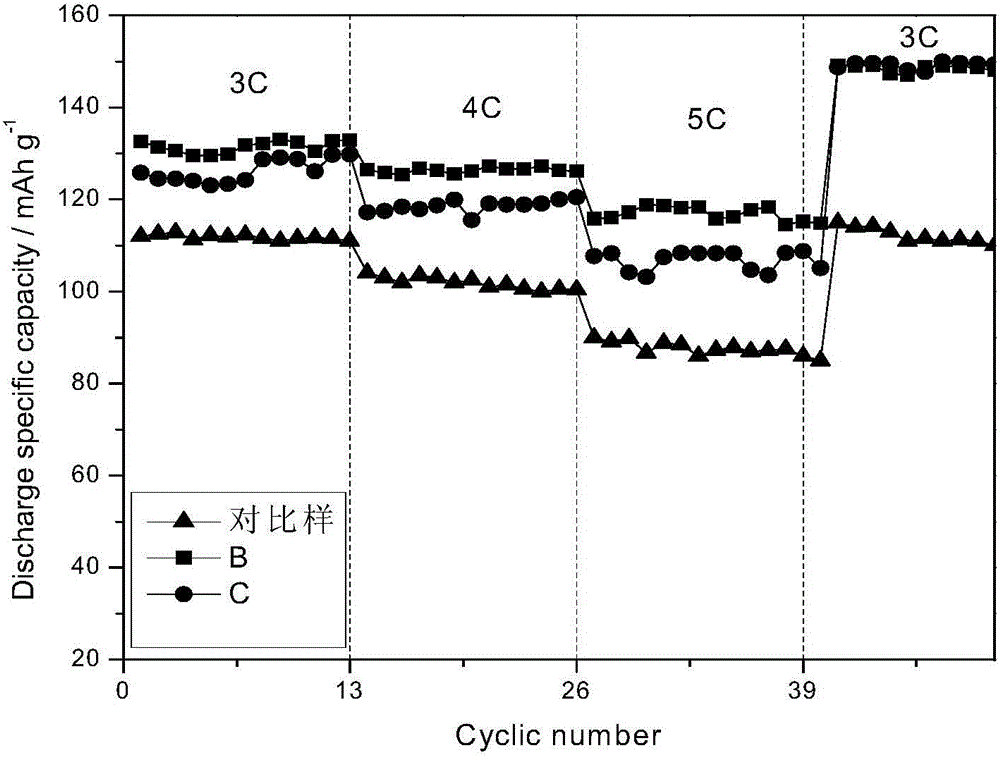
Preparation method for ultrafine impurity-doped ferrous oxalate special for lithium iron phosphate
A technology of lithium ferrous phosphate and ferrous oxalate is applied in the preparation of carboxylate, the preparation of carboxylate, the preparation of organic compounds, etc., which can solve the problem that the doping element is difficult to solidify, and achieves a simple preparation process, The effect of improving electrical conductivity and high-rate discharge performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Dissolve 170g of titanium dioxide by-product ferrous sulfate solid with a Fe content of 16.5% in tap water at a liquid-solid mass ratio of 1.5:1, stir, heat up to 90°C, and add ferrous sulfate with an average particle size of less than 74 μm and an iron content of more than 98%. Reduce the iron powder, keep the reaction pH at 4.5, and the reaction time is 10 hours. After the impurity removal reaction is completed, filter to obtain a pure ferrous sulfate solution.
[0021] 0.01molMgSO 4 .7H 2 O is added in the pure ferrous sulfate solution, then ammoniacal liquor is joined in the ferrous sulfate solution, and the ammoniacal liquor addition is 100% of theoretical amount, and feed time is 50 minutes, and 60 ℃ of reaction temperatures, promptly generate ferrous hydroxide precipitation at this moment, Heat preservation reaction for 0.5 hours. Make 68g of oxalic acid with a content of 99% into 200ml solution with tap water, drop it into the ferrous hydroxide suspension, the...
Embodiment 2
[0023] Dissolve 170g of titanium dioxide by-product ferrous sulfate solid with a Fe content of 16.5% in tap water at a ratio of liquid to solid mass ratio of 2.5:1, stir, heat up to 85°C, and add ferrous sulfate with an average particle size of less than 74 μm and an iron content of more than 98%. Reduce the iron powder, keep the reaction pH at 4.0, and the reaction time is 8 hours. After the impurity removal reaction is completed, filter to obtain a pure ferrous sulfate solution.
[0024] 0.003molCo(NO 3 ) 2 .6H 2 O is added in the pure ferrous sulfate solution, then ammoniacal liquor is joined in the ferrous sulfate solution, the ammoniacal liquor addition is 105% of theoretical amount, 10 minutes of feeding time, 50 ℃ of reaction temperature, promptly generate ferrous hydroxide precipitation at this moment, Heat preservation reaction for 0.5 hours. Make 68g of oxalic acid with a content of 99% into 200ml solution with tap water, drop it into the ferrous hydroxide suspens...
Embodiment 3
[0026] Dissolve 170g of titanium dioxide by-product ferrous sulfate solid with a Fe content of 16.5% in tap water at a liquid-to-solid mass ratio of 3.0:1, stir, heat up to 95°C, and add ferrous sulfate with an average particle size of less than 74 μm and an iron content greater than 98%. Reduce the iron powder, keep the reaction pH at 4.0, and the reaction time is 5 hours. After the impurity removal reaction is completed, filter to obtain a pure ferrous sulfate solution.
[0027] 0.001molCo(NO 3 ) 2 .6H 2 O and 0.007molMgCl 2 .6H 2 O is added in the pure ferrous sulfate solution, then ammoniacal liquor is joined in the ferrous sulfate solution, and the ammoniacal liquor addition is 105% of theoretical amount, and feed time is 30 minutes, and 90 ℃ of reaction temperatures, promptly generate ferrous hydroxide precipitation at this moment, Heat preservation reaction for 0.5 hours. Make 68g of oxalic acid with a content of 99% into 200ml solution with tap water, drop it into...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com