Hydroxyalkylated heterocycle boric acid ester and preparation method and purpose thereof
A technology of heterocyclic boronic acid ester and hydroxyalkylation, which is applied in the field of organic compounds and their preparation, and can solve problems such as strict requirements for lubricating additives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
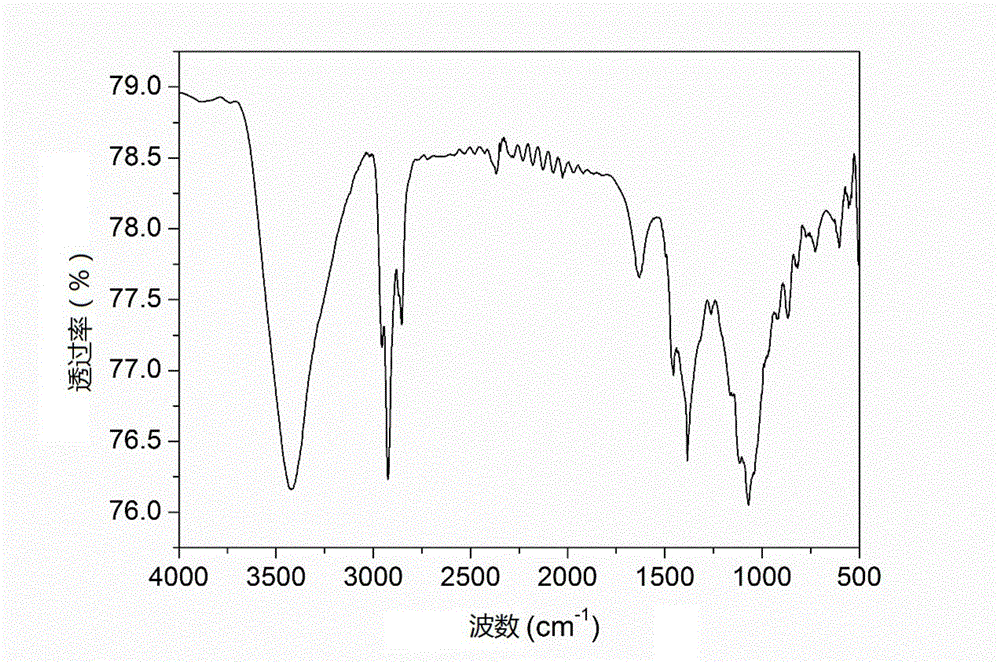
[0059] This embodiment relates to a hydroxyalkylated heterocyclic boronic acid ester (BPTT) shown in the following structural formula,
[0060]
[0061] This embodiment also relates to the preparation method of the aforementioned hydroxyalkylated heterocyclic boronic acid ester (BPTT), the method comprising the following steps:
[0062] Step 1: Add catalyst to a three-necked flask containing 15.0 g of 2,5-dimercaptothiadiazole, and after stirring at room temperature for half an hour, add 11.6 g of propylene oxide dropwise, and continue the reaction at room temperature overnight. After the reaction, the acid washing, washing with water, drying over anhydrous magnesium sulfate, filtering, and evaporating the organic solvent under reduced pressure to obtain 23.1 g of the intermediate product brownish-red oily liquid DMTO, with a yield of 86.8%;
[0063] Step 2: In a three-necked flask containing 35.8g N,N-dihydroxyethyl octadecylamine, 13.3g DMTO and 6.2g boric acid, add a cat...
Embodiment 2
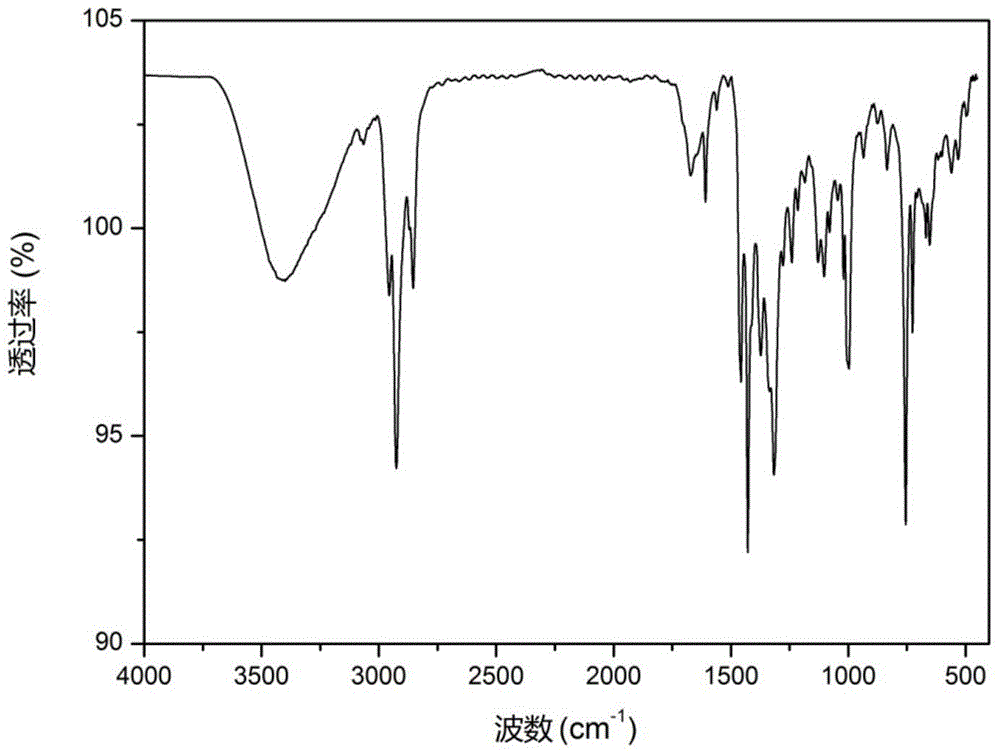
[0070] This embodiment relates to a hydroxyalkylated heterocyclic boronic acid ester (DBMT) shown in the following structural formula,
[0071]
[0072] This embodiment relates to the preparation method of the aforementioned hydroxyalkylated heterocyclic boronic acid ester (DBMT), and the method includes the following steps:
[0073] Step 1: Add a catalyst to a three-necked flask containing 16.7 g of 2-mercaptobenzothiazole, stir at room temperature for half an hour, add 6.0 g of propylene oxide dropwise, and continue to react overnight at room temperature. After the reaction is complete, wash with acid and water , dried over anhydrous magnesium sulfate, filtered, and the organic solvent was evaporated under reduced pressure to obtain 19.35 g of the intermediate product brownish-red oily liquid 2-benzothiazol-2-ylthio-1-methyl-ethanol (PBT), with a yield of 86.1 %;
[0074] Step 2: Add 11.61g p-dodecylbenzeneboronic acid and 18.03g PBT into a three-necked flask with toluen...
Embodiment 3
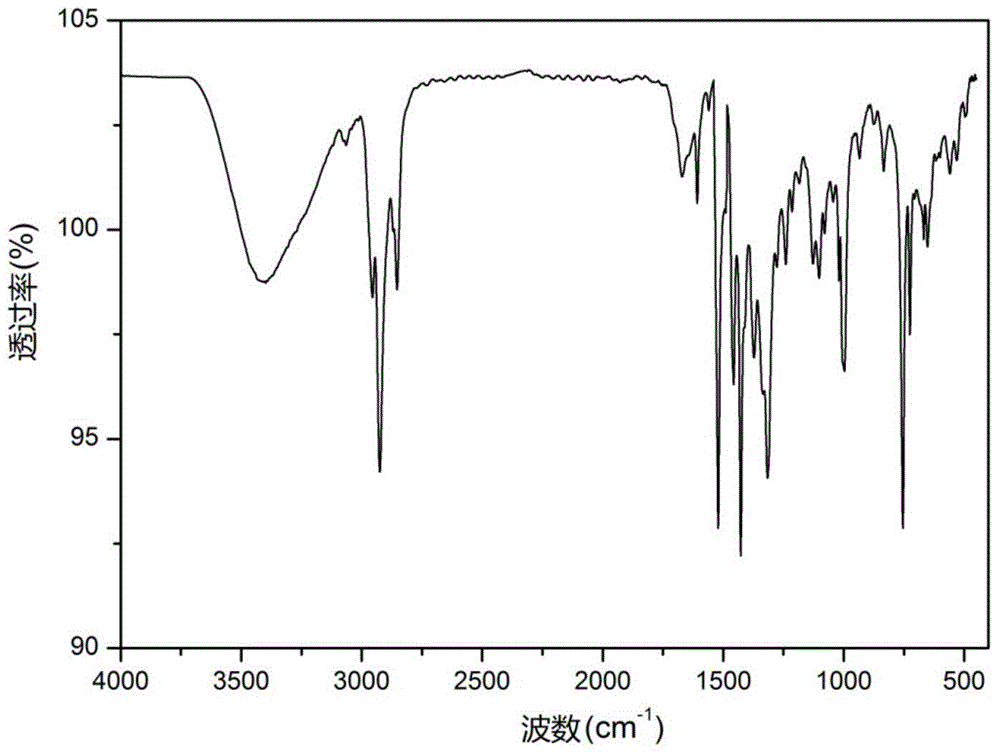
[0082] This example relates to a hydroxyalkylated heterocyclic boronic acid ester (DSSB) shown in the following structural formula,
[0083]
[0084] This embodiment relates to the preparation method of the aforementioned hydroxyalkylated heterocyclic boronic acid ester (DSSB), and the method includes the following steps:
[0085] Step 1, in a three-necked flask equipped with 17.73g of trimercaptotriazine, slowly add 50.0g of dodecane bromide dropwise, add 9g of sodium hydroxide as an acid-binding agent, overnight, add 6g of propylene oxide, and continue the reaction until the reaction Completely, the yellow oily intermediate DSEP45.76g was obtained, and the yield was 80.5%;
[0086] Step 2: Add 11.61g p-dodecylbenzeneboronic acid and 45.76g DSEP into a three-necked flask with toluene solvent, add catalyst, heat to reflux, and divide water for 8 hours. After the reaction, remove the organic solvent under reduced pressure to obtain DSSB is 53.13 g of hydroxyalkylated hetero...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com