Hydroxy-containing thiadiazole derivative, and preparation method and application thereof
A technology of hydroxythiadiazole and its derivatives, which is applied in the field of organic compounds and its preparation, can solve problems such as not meeting the requirements of environmental protection, achieve the effects of reducing the diameter of wear spots and enhancing the effect of extreme pressure and anti-wear
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
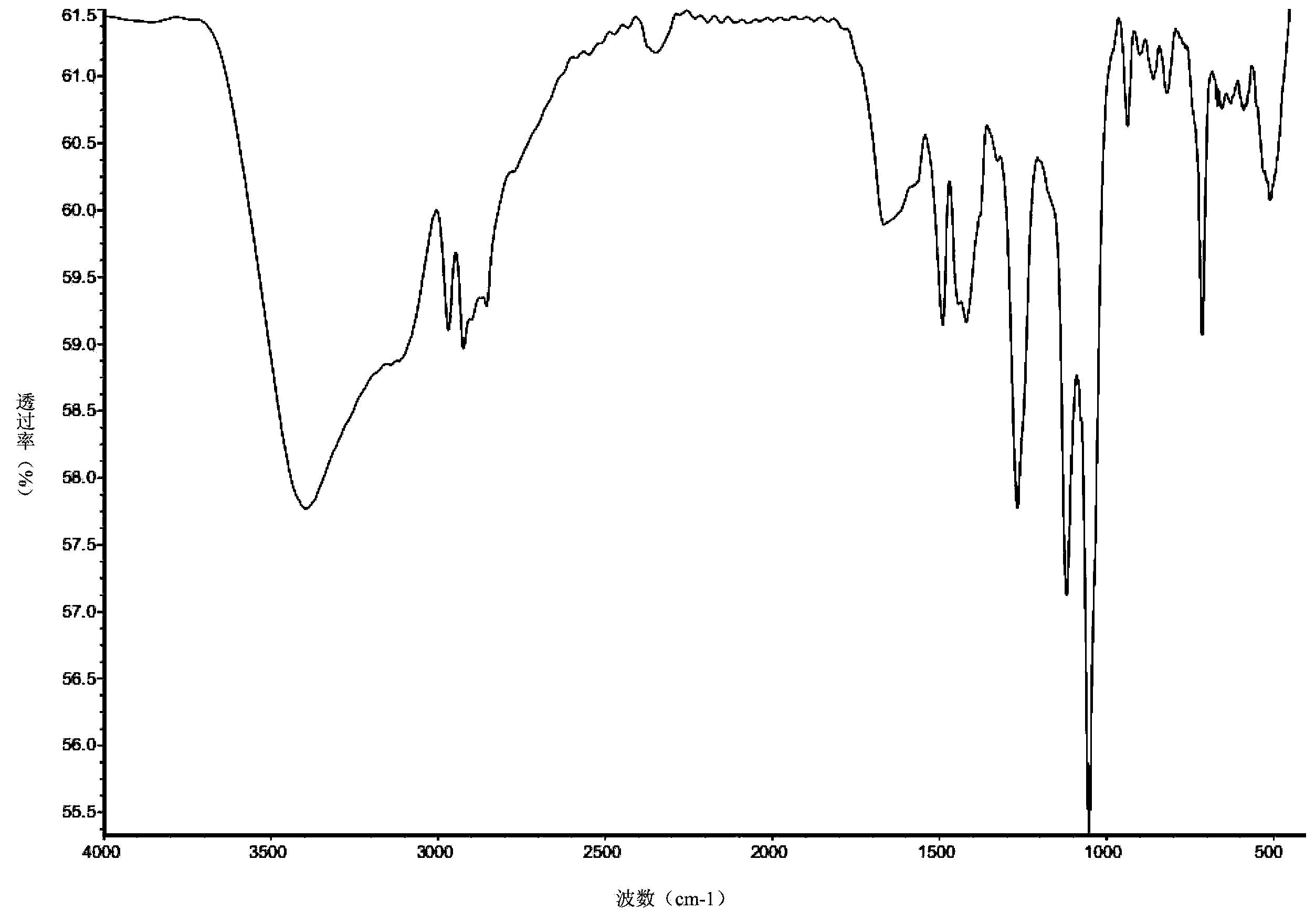
[0035] Take 7.5 g (0.05 mol) of 2,5-dimercapto-1,3,4-thiadiazole in a 150 ml three-necked flask, and add 50 ml of tetrahydrofuran as a solvent. After stirring at room temperature until uniformly mixed, 2.9 g (0.05 mol) of propylene oxide was slowly added dropwise. After the dropwise addition was completed, the temperature was raised to 80° C. to react for 24 hours. After the reaction, cool to room temperature, filter the reaction solution to remove insoluble matter, and then distill off the organic solvent to obtain the extreme pressure antiwear agent containing thiadiazole derivatives containing hydroxy. The product 2-(2'-hydroxypropyl)mercapto-5-mercapto-1,3,4-thiadiazole has a mass of 9.6 g and a yield of 92.3%. figure 1 Is the infrared spectrum analysis of the product, and the analysis results are as follows: IR (cm -1 ): 3399.27 (O-H), 2968.96, 2923.94 (-CH 3 、-CH 2 -), 1667.35 (C=N), 1489.04, 1419.20 (thiadiazole ring skeleton), 1265.86 (O-H), 1119.02, 1052.00 (C-O),...
Embodiment 2
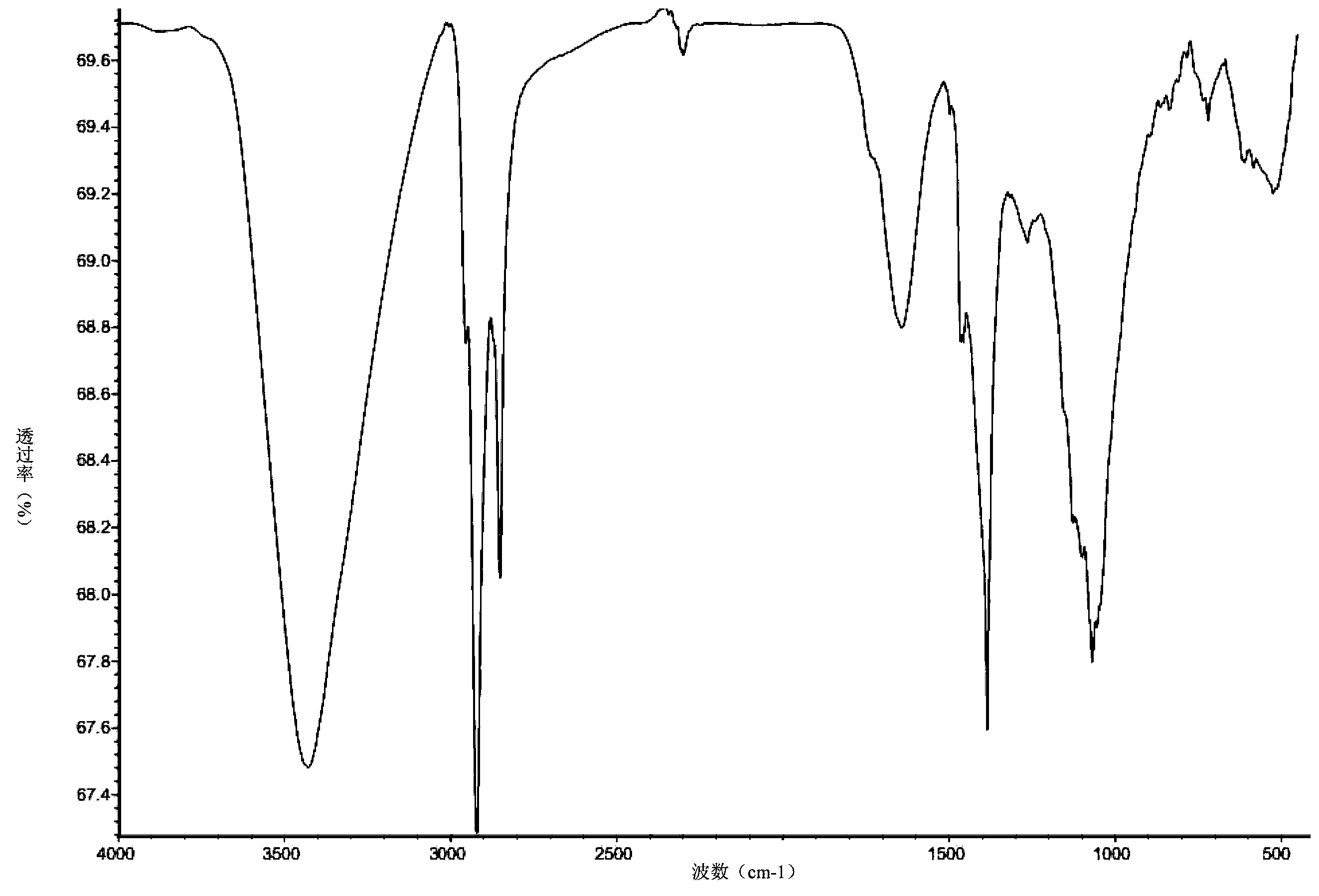
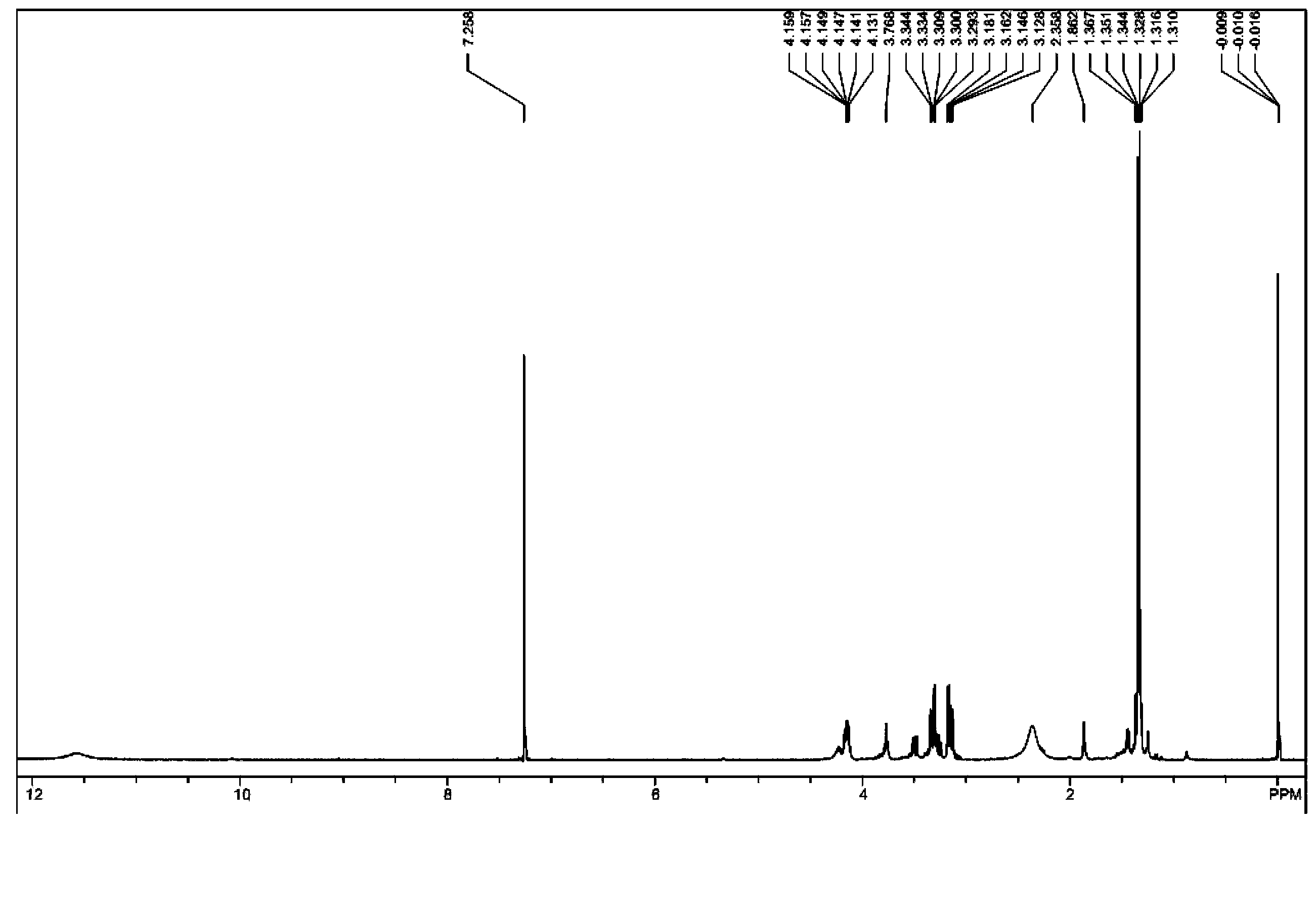
[0037] Take 7.5 g (0.05 mol) of 2,5-dimercapto-1,3,4-thiadiazole in a 150 ml three-necked flask, and add 50 ml of tetrahydrofuran as a solvent. After stirring at room temperature until uniformly mixed, 18.4 g (0.1 mole) of dodecane oxide was slowly added dropwise. After the dropwise addition was completed, the temperature was raised to 80° C. to react for 24 hours. After the reaction, cool to room temperature, filter the reaction solution to remove insoluble matter, and then distill off the organic solvent to obtain the extreme pressure antiwear agent containing thiadiazole derivatives containing hydroxy. The product 2,5-bis(2'-hydroxydodecyl)mercapto-1,3,4-thiadiazole has a mass of 24.5 grams and a yield of 94.6%. figure 2 Is the infrared spectrum analysis of the product, and the analysis results are as follows: IR (cm -1 ): 3428.68 (O-H), 2920.83, 2851.26 (-CH 3 、-CH 2 -), 1642.36 (C=N), 1384.75 (-CH 3 ), 1068.25 (C-O), 719.75 (C-S). Figure 4 Be the proton nuclear ma...
Embodiment 3
[0041] This example is an elemental analysis of a sample. Table 1 has listed the elemental analysis result of embodiment 1~2. From the elemental analysis results in Table 1, it can be seen that the measured values of C, H, N, and S elements of all target compounds and the theoretical values calculated according to the molecular formula basically meet the requirements of grease additives, and the absolute error is within the allowable range. It can be confirmed that the obtained compound is the target compound.
[0042] Table 1 elemental analysis results
[0043]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com