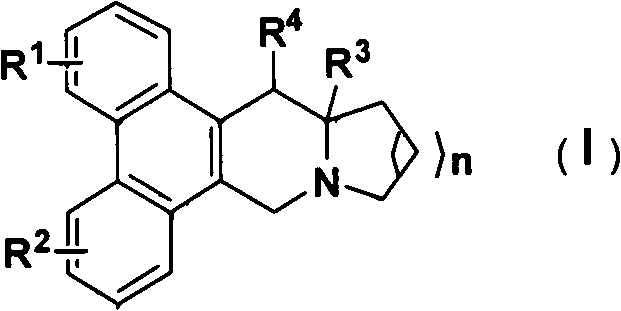
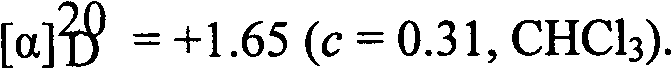Phenanthroindolizidine (or phenanthroquinolizidine) alkaloid derivatives, preparation methods and anti-plant virus activities thereof
A technology of phenanthrene and indolizidine and alkaloid derivatives, which is applied in the field of preparation and anti-plant virus activity, can solve the problems of less research, and achieve good anti-plant virus activity and excellent anti-plant virus activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
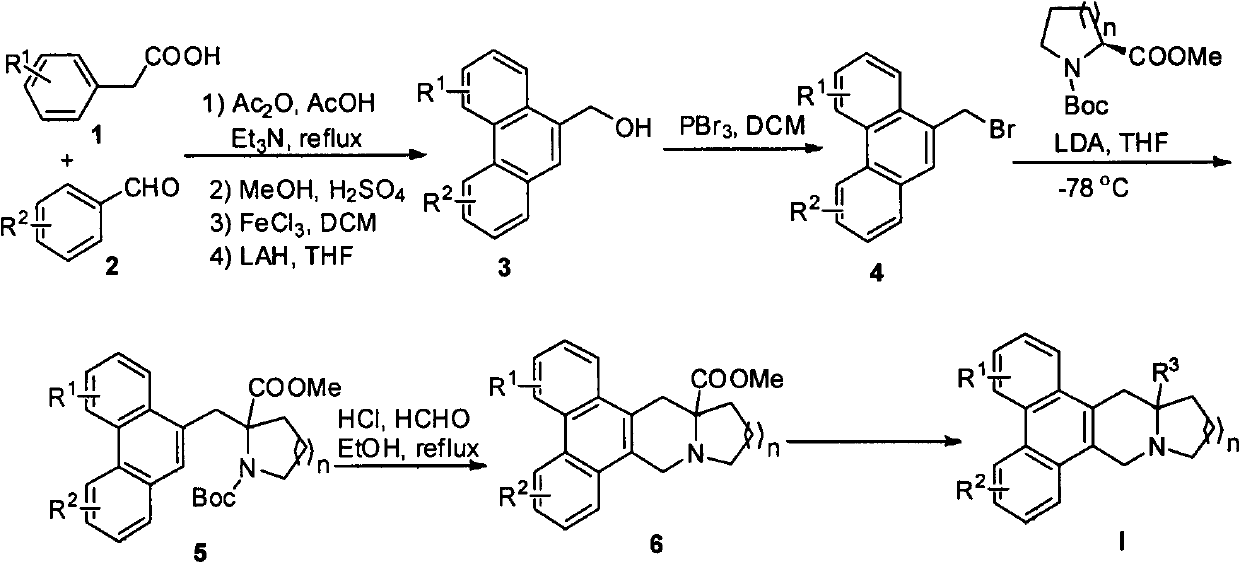
[0031] Example 1: 13a substituted phenanthroindolizidine and 14a substituted quinolizidine alkaloid derivatives I-1~I-3, I-15~I-17 and I-31~I-32 Synthesis
[0032] Synthesis of 13a-substituted phenanthrene indolizidine alkaloid derivatives I-1, I-2 and I-3:
[0033]
[0034] Synthesis of 1-tert-butyl formate-2-methyl formate-2-(2,3,7-trimethoxyphenanthrenemethyl)pyrrolidine: Add phenanthrene methanol (1.79 g, 6 mmol) in a 250 mL round bottom flask and dichloromethane, and then dropwise added phosphorus tribromide (3.25g, 12mmol) with a constant pressure dropping funnel. After the reaction was completed, water was added to the reaction system to quench the reaction. The liquid was separated, and the organic phase was washed with ice water, and the organic phase obtained from the liquid separation was evaporated by rotary evaporation to obtain the phenanthrene methyl bromide. The phenanthrene methyl bromide was carried on to the next step without further purification. In ...
Embodiment 2
[0043] Example 2: 13a substituted phenanthroindolizidine and 14a substituted quinolizidine alkaloid derivatives I-4, I-11~I-14, I-18, I-22~I-27 and the synthesis of I-33
[0044] Synthesis of Compounds I-4, I-11, I-12 and I-14:
[0045]
[0046] Synthesis of I-11: Add ethylene glycol (40mL), raw material ester I-1 (0.48g, 1.1mmol), hydrazine hydrate (80%, 2mL) successively in a 100mL reaction flask, heat to reflux for 2 hours, stop To react, dichloromethane (80 mL) was added to dilute the reaction, washed with water (20 mL*3), washed with saturated brine, dried over anhydrous sodium sulfate, and precipitated to obtain I-1 (0.44 g, 94%): melting point: 210 ° C decomposition; 1 HNMR (400MHz, CDCl 3 )δ8.68(s, 1H), 7.92-7.76(m, 3H), 7.42(s, 1H), 7.18(d, J=7.8Hz, 1H), 4.21(d, J=15.8Hz, 1H), 4.16(d, J=15.8Hz, 1H), 4.08(s, 3H), 4.07(s, 3H), 3.98(s, 3H), 3.82(s, 1H), 3.73(s, 1H), 3.40(d , J=15.6Hz, 1H), 3.22(s, 1H), 3.17(d, J=15.6Hz, 1H), 2.57(dd, J=16.4, 8.4Hz, 1H), 2.30-2.23(...
Embodiment 3
[0054] Example 3: 13a substituted phenanthroindolizidine and 14a substituted quinolizidine alkaloid derivatives I-5~I-9, I-19~I-22, I-28~I-30 Synthesis with I-34~I-39
[0055] Synthesis of Compounds I-19, I-22 and I-28:
[0056]
[0057] Synthesis of I-22: Add dichloromethane (40mL), raw material alcohol I-16 (0.40g, 0.95mmol), DMAP (0.14g, 1.1mmol) in a 100mL reaction flask, slowly add acetyl chloride (0.08 g, 1.0 mmol) in dichloromethane (10 mL), the dropwise addition was completed, and the stirring reaction was continued for 2 hours. Precipitate under reduced pressure, add water (30mL), add saturated sodium hydroxide solution dropwise, adjust pH to 14, extract with dichloromethane (30mL*3), combine organic phases, wash with saturated brine, dry over anhydrous sodium sulfate, remove A brown-yellow solid was dissolved and separated by column chromatography to obtain a yellow solid (0.35g, 80%): melting point 167-170°C; 1 HNMR (400MHz, CDCl 3 )δ7.85(s, 1H), 7.85(s, 1H),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com