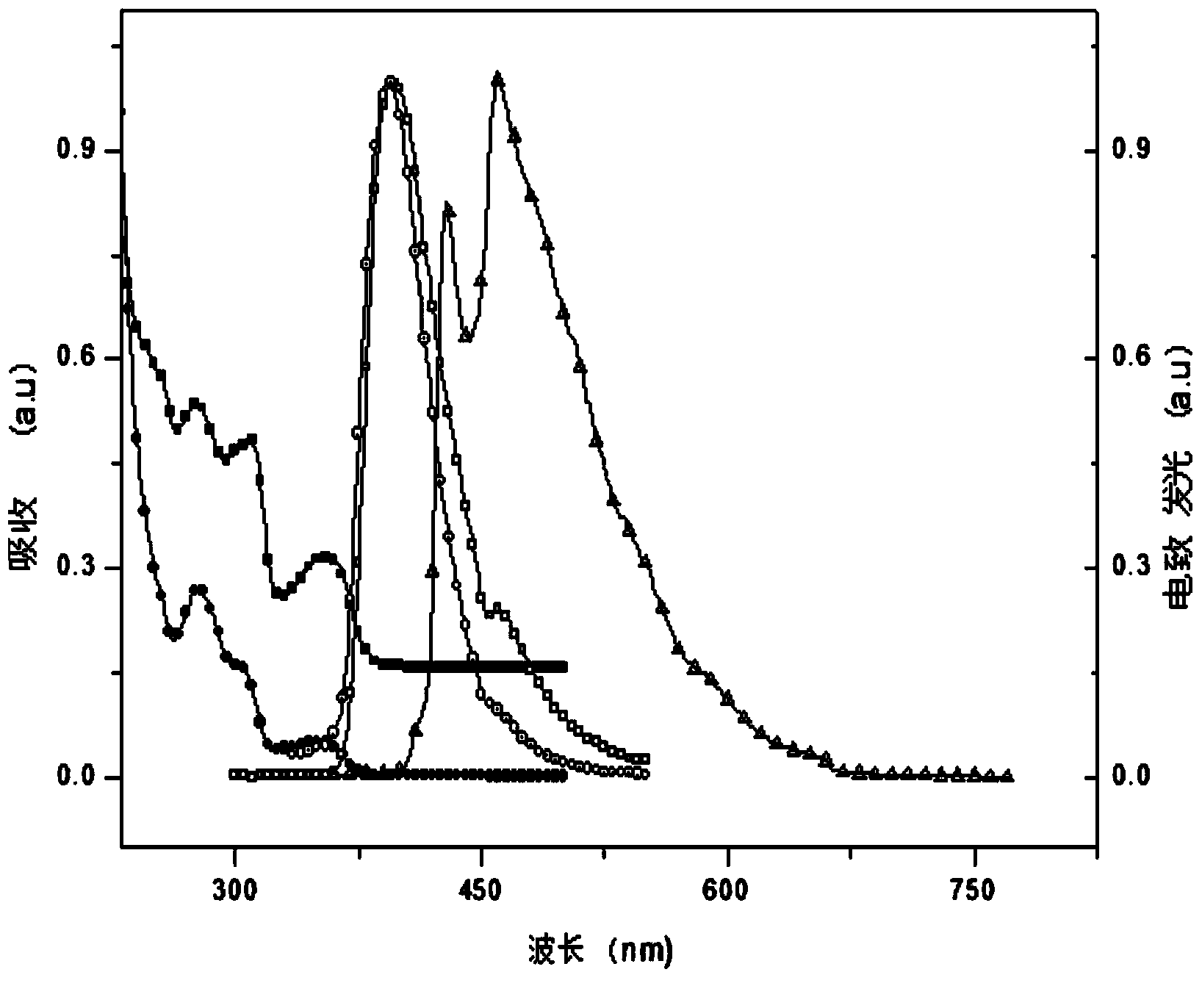
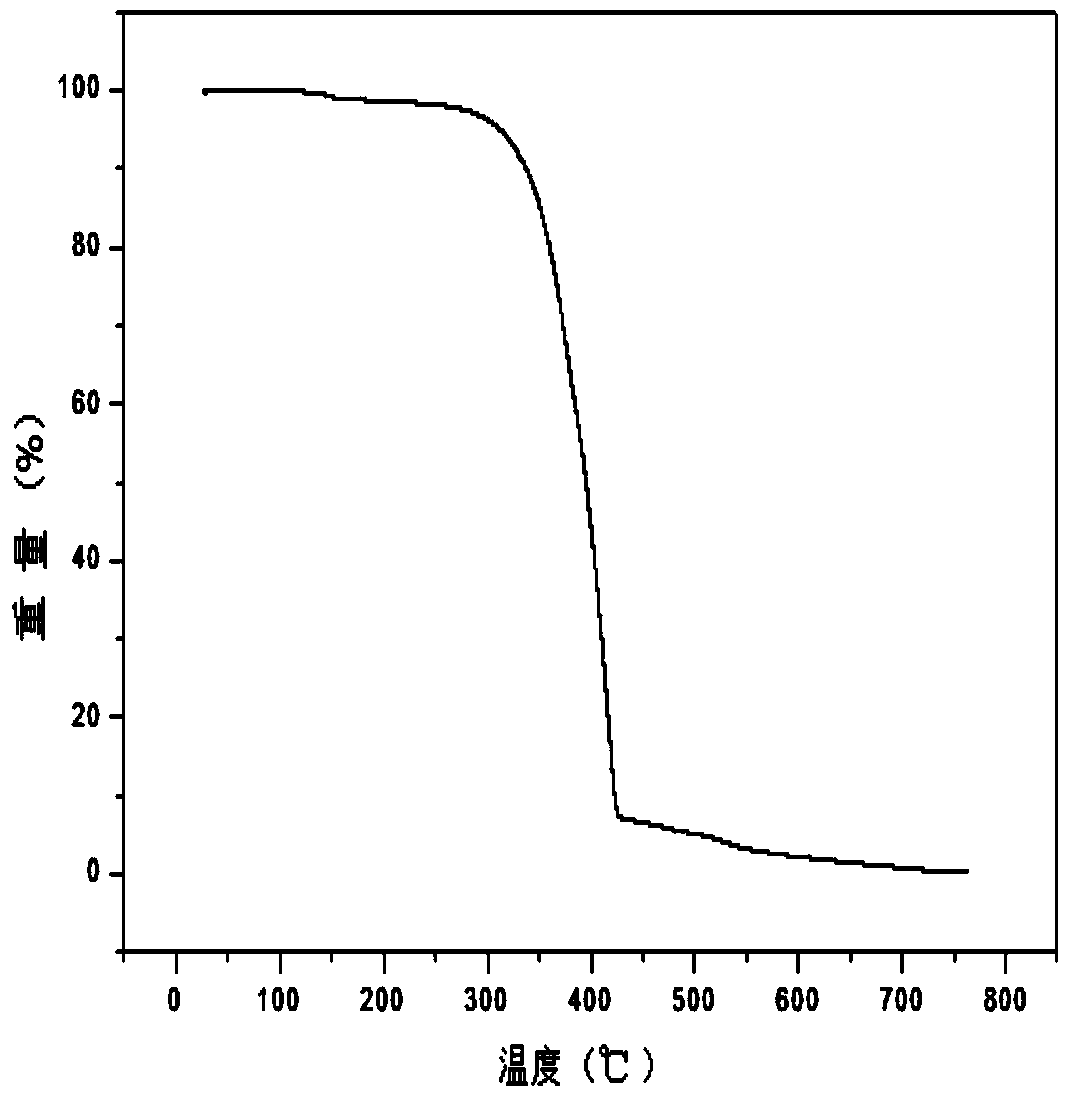
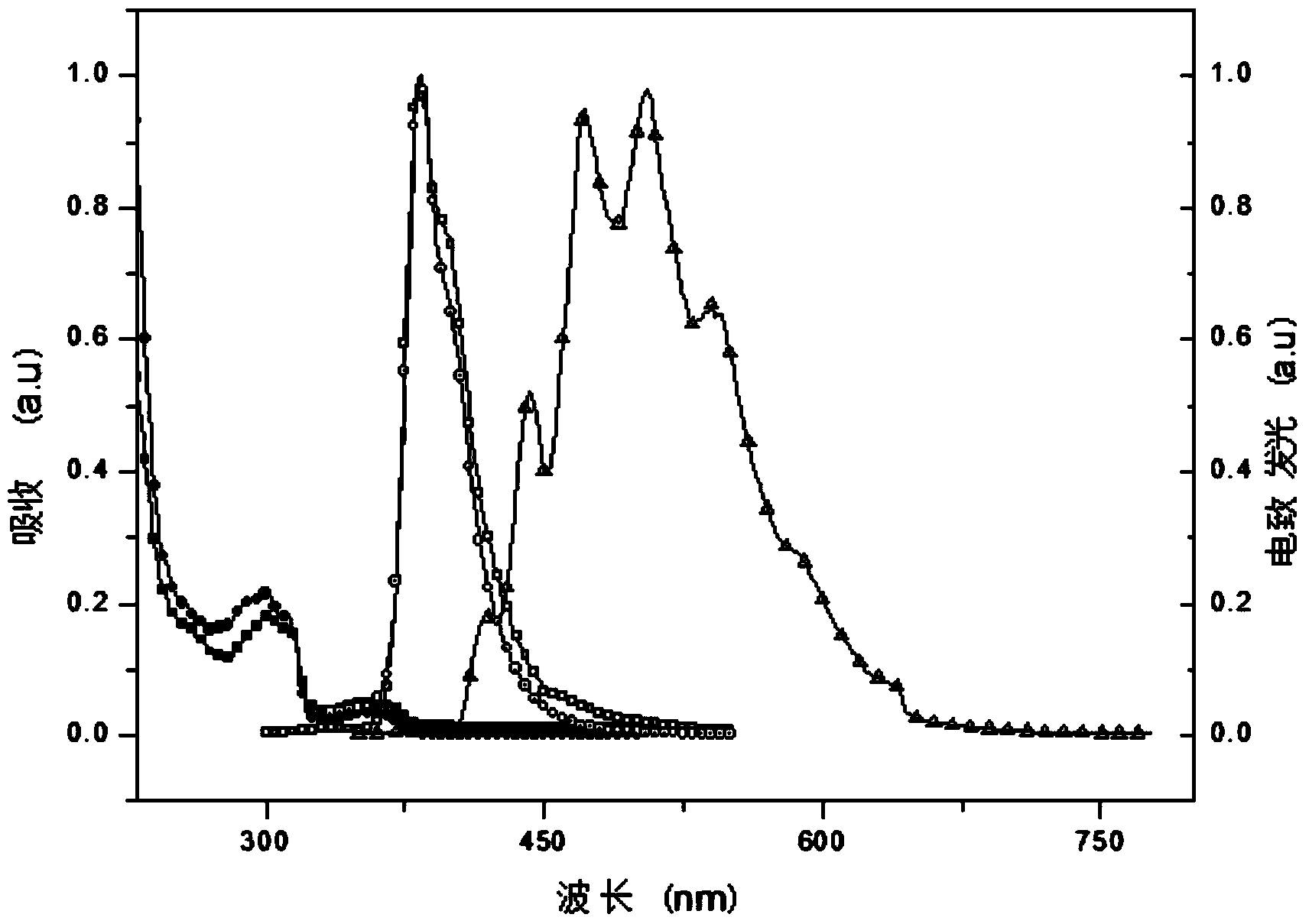
Multifunctional modified tert-butyl carbazole phosphine oxide main material and synthesis method and application thereof
A technology of tert-butyl carbazole phosphine and tert-butyl carbazole, which is applied in the field of multifunctional modified tert-butyl carbazole phosphine oxygen host material, synthesis and application, and can solve the problems of high turn-on voltage, luminous efficiency and brightness Low-level problems, to achieve the effects of improving luminous efficiency and brightness, good thermodynamic stability, and enhancing carrier injection and transport capabilities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0067] Specific embodiment 1: The method for synthesizing the multifunctionally modified tert-butyl carbazole phosphine oxide host material of this embodiment is implemented in the following steps:
[0068] 1. Combine 1mmol of carbazole, 2~5mmol of chloro-tert-butane, 50~100ml of chloroform and 0.5~1.5mmol of anhydrous AlCl 3 Stir and react for 5-24 hours, then pour into ice water, and use HCl solution and saturated NaHCO 3 And CH 2 Cl 2 The organic phase is obtained by extraction, and the obtained organic phase is dried and acidified with sulfuric acid, and then saturated NaHCO 3 The organic layer is obtained by extraction, and after drying, a mixed solvent of petroleum ether and ethyl acetate is used as an eluent for purification to obtain tert-butylcarbazole;
[0069] 2. Dissolve the tert-butylcarbazole synthesized in step 1 in N,N-dimethylformamide (DMF), and the molar ratio of tert-butylcarbazole to N-bromosuccinimide is 1: (2~3) Add N-bromosuccinimide (NBS), react for 1~10 hou...
specific Embodiment approach 2
[0073] Embodiment 2: The method for synthesizing the multifunctional modified tert-butyl carbazole phosphine oxide host material of this embodiment is implemented in the following steps:
[0074] 1. Combine 1mmol of carbazole, 2~5mmol of chloro-tert-butane, 50~100ml of chloroform and 0.5~1.5mmol of anhydrous AlCl 3 Stir and react for 5-24 hours, then pour into ice water, and use HCl solution and saturated NaHCO 3 And CH 2 Cl 2 The organic phase is obtained by extraction, and the obtained organic phase is dried and acidified with sulfuric acid, and then saturated NaHCO 3 The organic layer is obtained by extraction, and after drying, a mixed solvent of petroleum ether and ethyl acetate is used as an eluent for purification to obtain tert-butylcarbazole;
[0075] 2. Dissolve the tert-butylcarbazole synthesized in step 1 in N,N-dimethylformamide (DMF), and the molar ratio of tert-butylcarbazole to N-bromosuccinimide is 1: (1~3) Add N-bromosuccinimide (NBS), react for 1~10 hours, pour in...
specific Embodiment approach 3
[0081] Specific embodiment three: This embodiment is different from the specific embodiment two in that the molar ratio of step three is 1:1.5:0.05:5. The bromo-tert-butylcarbazole, anhydrous K 2 CO 3 , CuI and bromobenzene mixed. Other steps and parameters are the same as in the second embodiment.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com