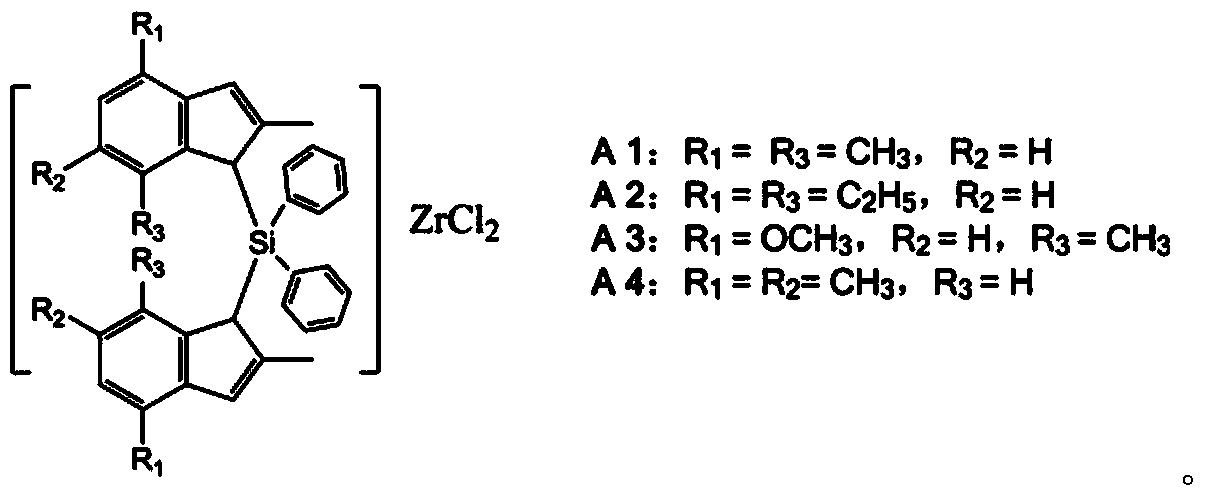Bridged metallocene complex and its application
A technology of metallocene complexes and metallocene catalysts, applied in metallocene, organic chemistry, chemical instruments and methods, etc., can solve problems such as the inability to control the performance of ethylene-propylene rubber, and achieve the effect of strong copolymerization catalytic ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Synthesis of bridged metallocene complex A1:
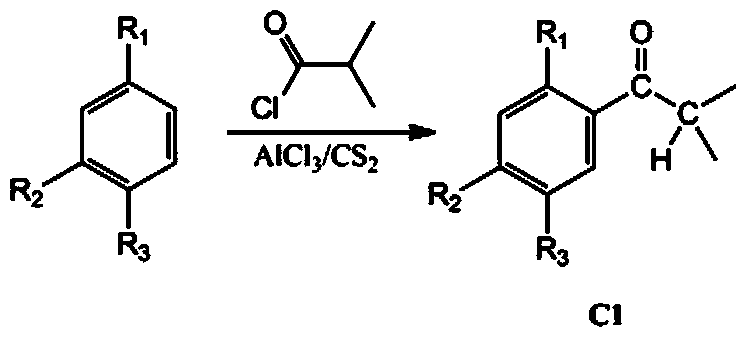
[0045] (1) Synthesis of 1-(2,5-dimethylphenyl)-2-methylpropan-1-one: 78.6 g (0.74 mol) p-xylene, 61 g (0.45 mol) anhydrous AlCl 3 The mixture of the powder and 500mL of carbon disulfide was cooled to 0°C, and 79.4g (0.74mol) of methacryloyl chloride was slowly added dropwise to the mixture while stirring. After the temperature of the mixture was raised to room temperature, stirring was continued for 12 hours. Then add 61 g (0.45 mol) of anhydrous AlCl 3 powder and 250mL carbon disulfide. The mixture was refluxed at 45°C for 3 hours, cooled to room temperature and poured into an ice / hydrochloric acid mixture. The organic phase is separated and the carbon disulfide is removed by gas flow. The remaining liquid was extracted with ether and washed with anhydrous Na 2 SO 4 After drying, 93.0 g of 1-(2,5-dimethylphenyl)-2-methylpropan-1-one were obtained, with a yield of 71%. 1 H-NMR (300MHz, CDCl 3 ,δin ppm):7.57(s,1H,Ar-...
Embodiment 2
[0053] Synthesis of bridged metallocene complex A2:
[0054] (1) Synthesis of 1-(2,5-diethylphenyl)-2-methylpropan-1-one: the reaction conditions are the same as those of 1-(2,5-dimethylphenyl)-2 in Example 1 -Synthesis of methylpropan-1-one, only by replacing p-xylene with p-diethylbenzene, to obtain 91.3 g of 1-(2,5-dimethylphenyl)-2-methylpropan-1-one, Yield 59%. 1 H-NMR (300MHz, CDCl 3 ,δin ppm):7.70(s,1H,Ar-H),7.25(s,1H,Ar-H),7.15(s,1H,Ar-H),3.34(sept,1H,CH(CH 3 ) 2 ),2.59(s,4H,Ar-CH 2 ),1.23(d,6H,CHCH 3 ). Elem.Anal.Calcd.For C 14 h 20 O: C, 82.35%; H, 9.80%; O, 7.84%. Found: C, 82.30%; H, 9.84%; O, 7.86%. ESI-MS: m / z205.10 ([M+H] + ).
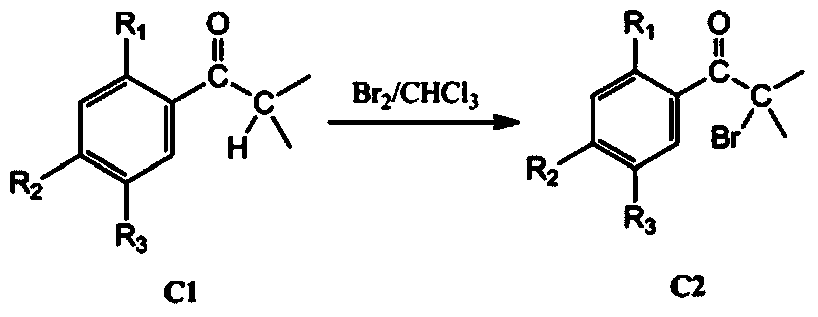
[0055] (2) Synthesis of α-bromo-2,5-diethylisobutyrophenone: the reaction conditions are the same as the synthesis of α-bromo-2,5-dimethylisobutyrophenone in Example 1, only 1-( 2,5-Dimethylphenyl)-2-methylpropan-1-one was replaced by 1-(2,5-diethylphenyl)-2-methylpropan-1-one to give 69.9 g of α -Bromo-2,5-dimethylisobutyr...
Embodiment 3
[0062] Synthesis of bridged metallocene complex A3:
[0063] (1) Synthesis of 1-(2-methoxy-5-methylphenyl)-2-methylpropan-1-one: the reaction conditions are the same as those of 1-(2,5-dimethylphenyl) in Example 1 )-2-methylpropan-1-one, only p-xylene was replaced by p-methylanisole, and 72.8 g of 1-(2-methoxy-5-methylphenyl)-2- Methylpropan-1-one, 51% yield. 1 H-NMR (300MHz, CDCl 3 ,δin ppm):7.58(s,1H,Ar-H),7.13(s,1H,Ar-H),6.73(s,1H,Ar-H),3.73(s,3H,OCH 3 ),3.34(sept,1H,CH(CH 3 ) 2 ),2.35(s,3H,Ar-CH 3 ),1.23(d,6H,CHCH 3 ). Elem.Anal.Calcd.For C 12 h 16 o 2 : C, 75.0%; H, 8.33%; O, 16.67%. Found: C, 74.95%; H, 8.31%; O, 16.74%. ESI-MS: m / z193.10 ([M+H] + ).
[0064] (2) Synthesis of α-bromo-2-methoxy-5-methylisobutyrophenone: the reaction conditions are the same as the synthesis of α-bromo-2,5-dimethylisobutyrophenone in Example 1, only 1-(2,5-Dimethylphenyl)-2-methylpropan-1-one was replaced by 1-(2-methoxy-5-methylphenyl)-2-methylpropane-1- ketone to obtain 64...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com