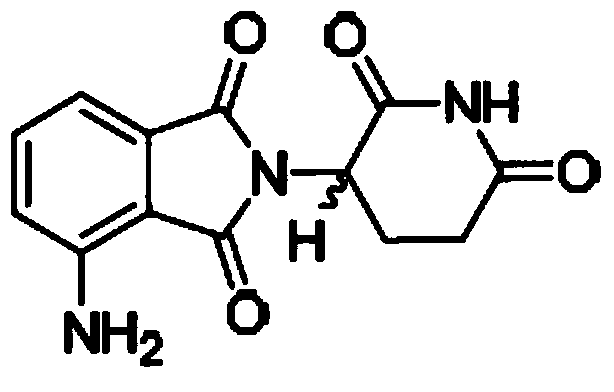
Method for simultaneously determining 4-amino-2-(2,6-dioxo-3-piperidyl)isoindoline-1,3-dione and related substances thereof
A technology for pomalidomide and related substances, applied in the field of determination and separation of pomalidomide and its related substances, can solve the problems of difficult impurities, difficult quality control, hidden dangers of safe drug use, etc., and achieve good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The preparation of embodiment 1 system suitability solution
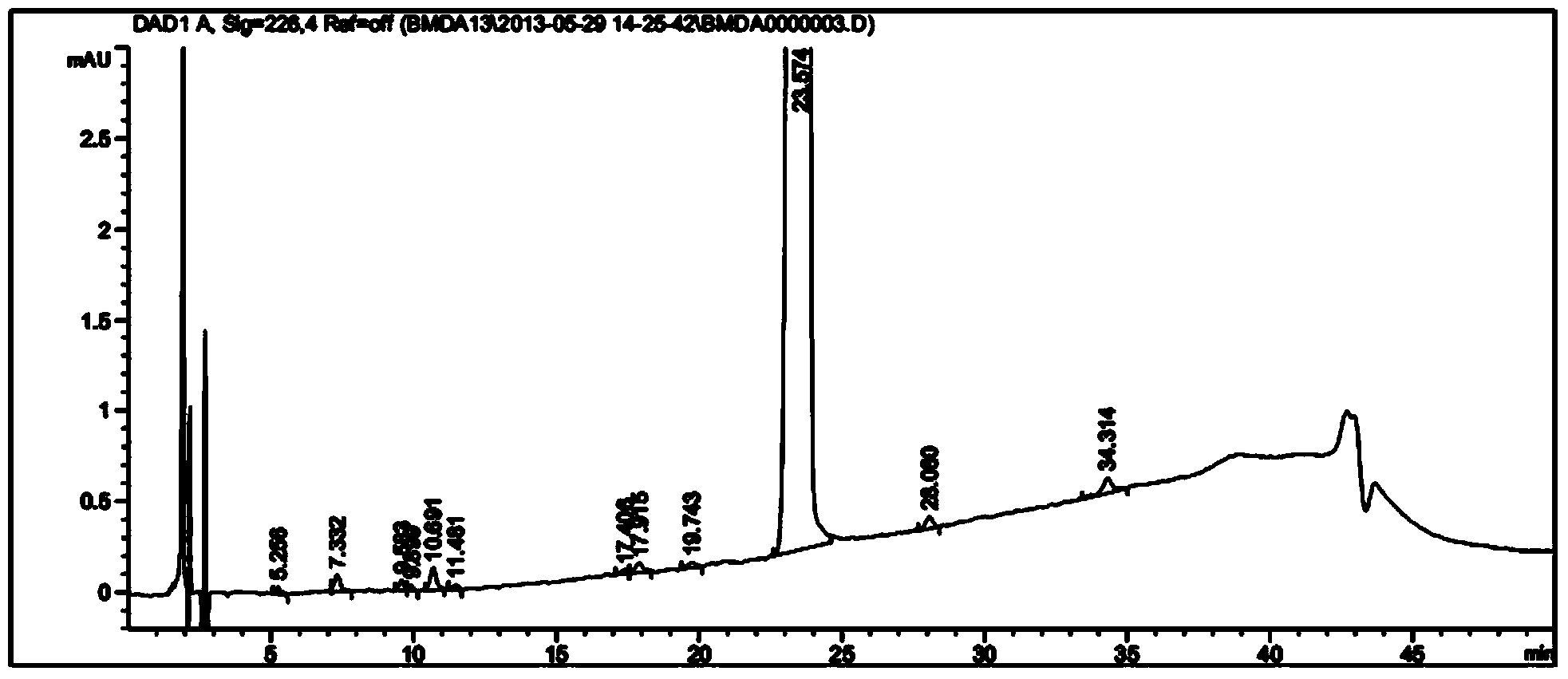
[0031] Take pomalidomide and by-product 2: 5-amino-2-(2,6-dioxo-piperidin-3-yl)-isoindoline-1,3-dione; appropriate amount, use acetonitrile Dissolve in an appropriate amount, add mobile phase A with acetic acid with a pH value of 2.6 to 3.0, and dilute to a solution containing both pomalidomide and by-product 2 at 10 μg / ml. The final volume ratio of acetonitrile and mobile phase A with a pH value of 2.6 to 3.0 acetic acid is 20:80. By-product 2 is the by-product closest to pomalidomide, and when it is separated from by-product 2, other impurities can be separated.
Embodiment 2
[0032] The screening of embodiment 2 chromatographic conditions and system suitability
[0033] 2.1 Selection of mobile phase
[0034] According to the relevant requirements of the mass spectrometry detector, the separation effects under different mobile phase conditions are as follows (see Table 1); the acetic acid solution is water to which acetic acid is added to adjust the pH.
[0035] Table 1. Mobile phase selection results
[0036] mobile phase
seperate effect
Methanol-acetic acid solution (pH2.2) (30:70)
The resolution is 4.3
Methanol-acetic acid solution (pH2.6) (30:70)
Separation is 8.5
Methanol-acetic acid solution (pH3.0) (30:70)
Separation is 6.1
Methanol-acetic acid solution (pH2.6) (gradient 1)
Resolution is 11.2
Methanol-acetic acid solution (pH2.6) (gradient 2)
The resolution is 9.2
[0037] According to the separation results of pomalidomide peak and by-product 2 peak in the system su...
Embodiment 3
[0054] The preparation of embodiment 3 detection limit solution
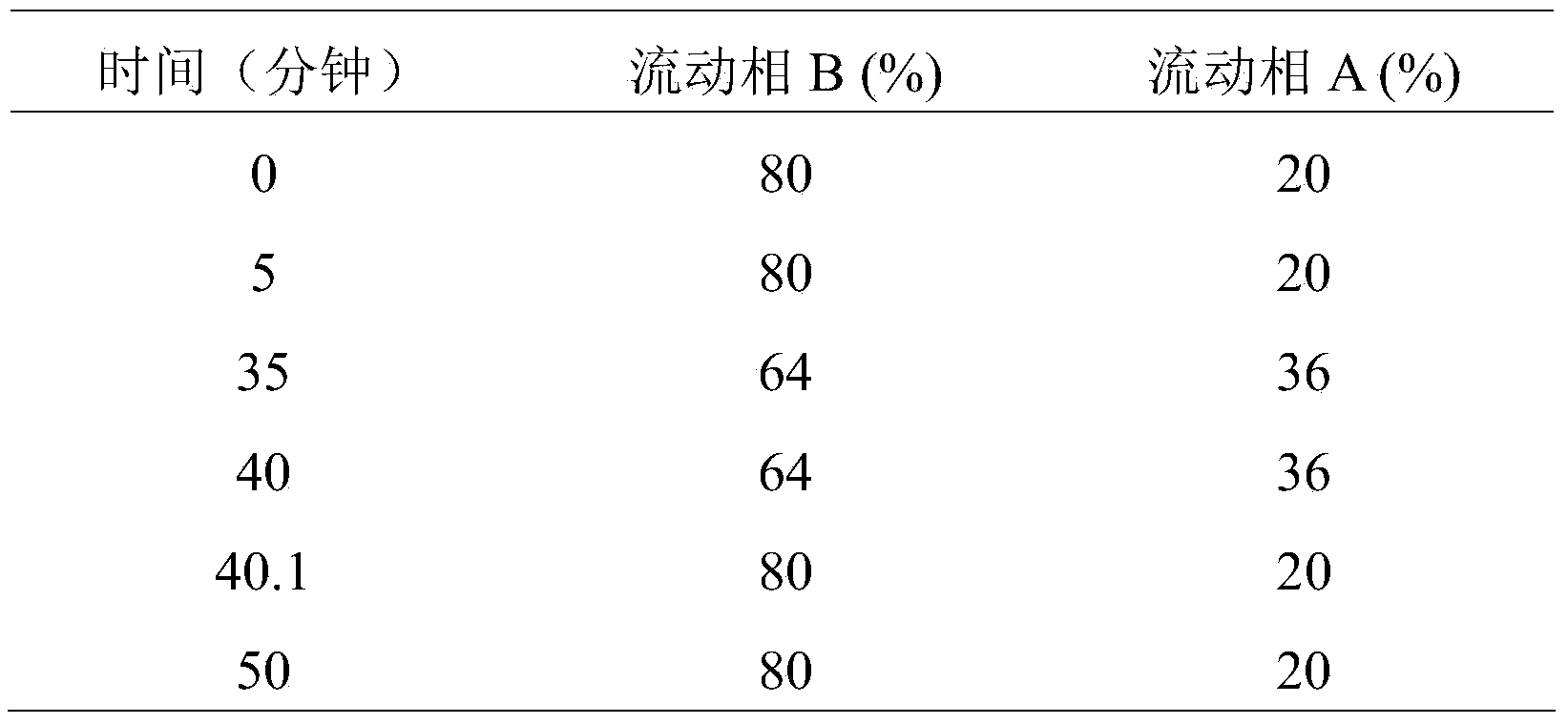
[0055] Detection limit solution: Accurately measure 0.11ml of the test solution, put it in a 100ml measuring bottle, add mobile phase A-mobile phase B (80:20 (v / v)) to dilute to the mark, shake well, and get it.
[0056] System suitability solution: take an appropriate amount of pomalidomide and by-product 2, dissolve them in an appropriate amount of acetonitrile, and dilute with mobile phase B to a solution containing pomalidomide and by-product 2 respectively at 10 μg / ml.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com