Method for removing carbon dioxide from gas mixture through biphasic absorption
A gas mixture and carbon dioxide technology, applied in chemical instruments and methods, separation methods, chemical separation, etc., can solve the problems of small absorption capacity of diethanolamine, difficult separation of two phases, high desorption temperature of heavy phase, etc., to achieve industrial production, Effect of low regeneration temperature and improved absorption rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
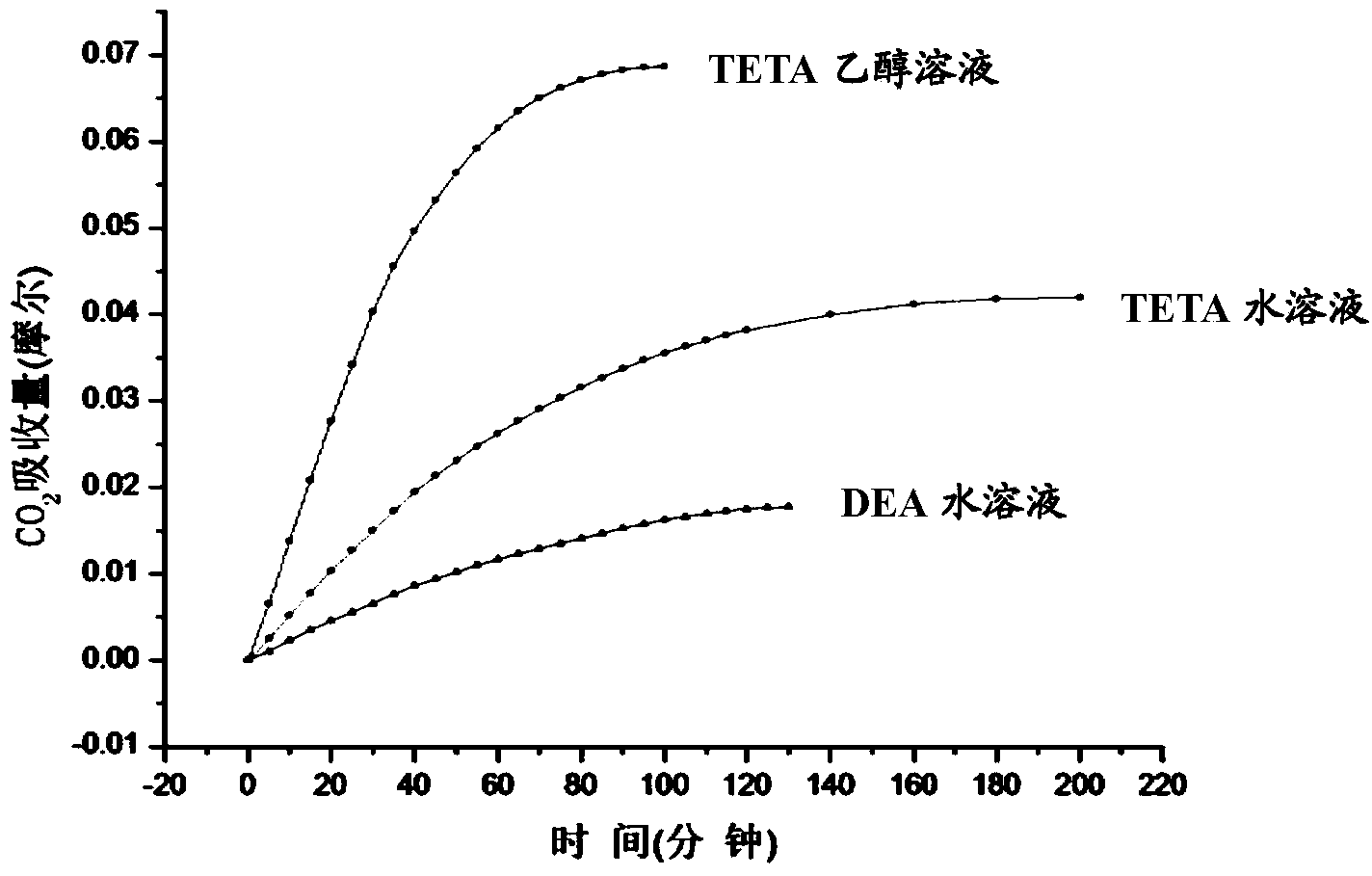
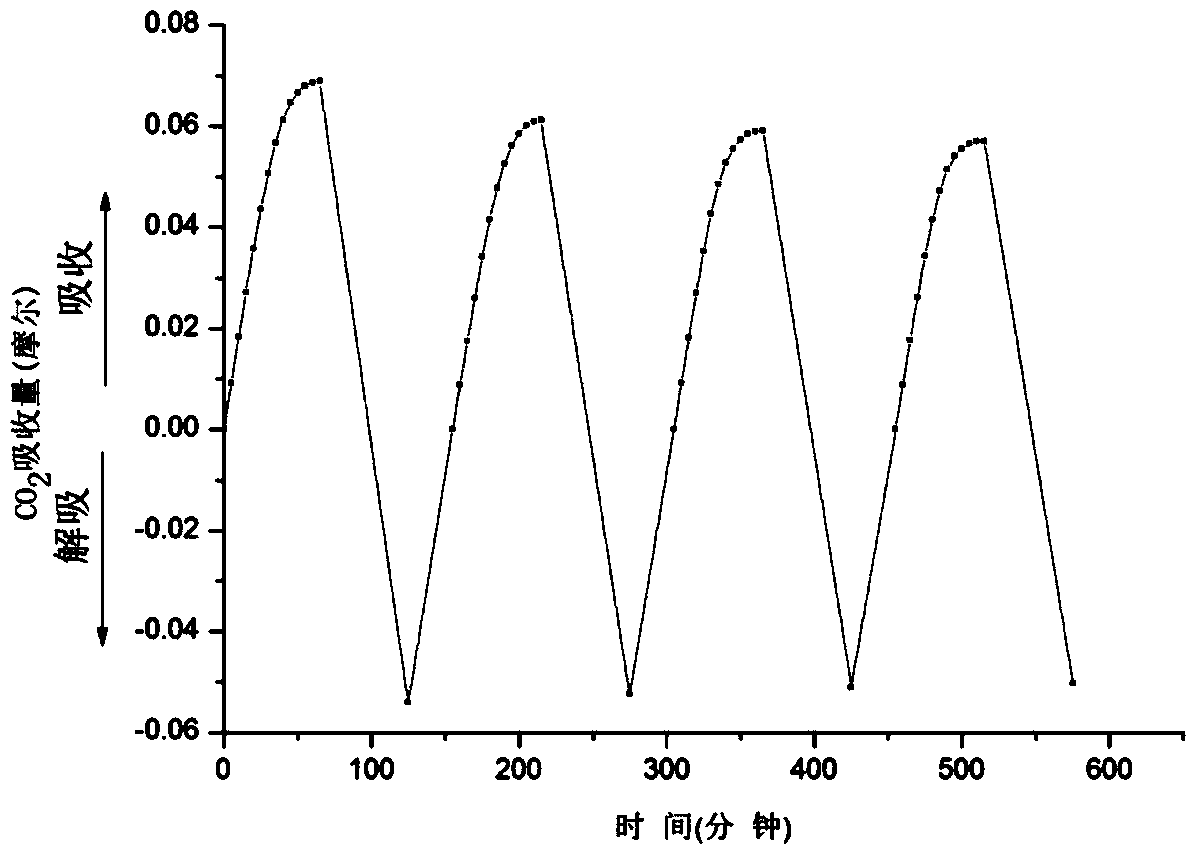
[0034] Add 200ml of triethylenetetramine (TETA) ethanol solution with a concentration of 0.2M into a double stirred tank with a height of 15cm and an inner diameter of 8cm. For the mixed gas of carbon dioxide, the inlet flow rate is controlled by a mass flowmeter, and the outlet flow rate is measured by a soap film flowmeter, which is measured every five minutes. The instantaneous absorption rate is calculated by the flow difference between the inlet and outlet, and the absorption amount is obtained by integrating the absorption rate with time, see figure 1 Middle curve A, its maximum absorption capacity is 0.068mol CO 2 .
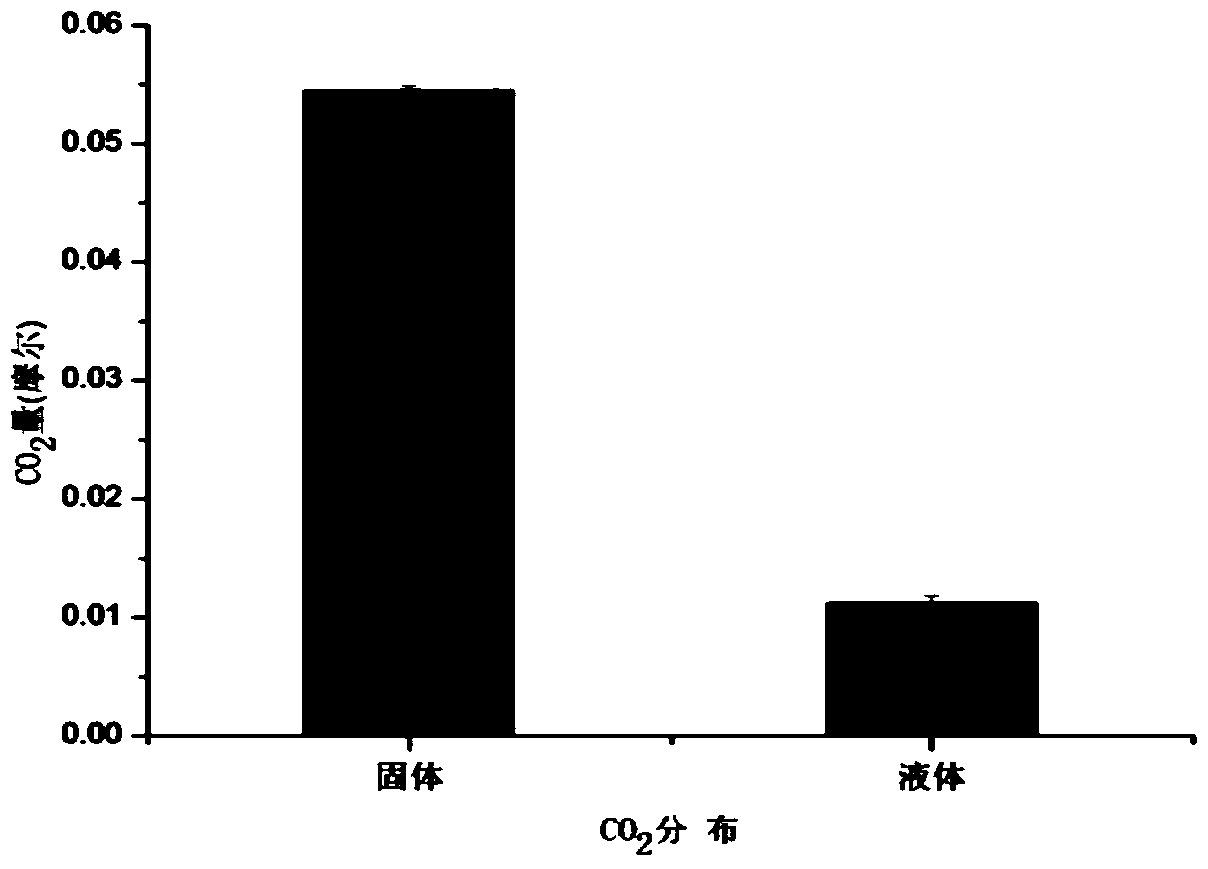
[0035] After the absorption is completed, separate the solid phase and the liquid phase through the suction filter funnel, transfer the solid phase and the liquid phase to the flask respectively, slowly drop in dilute sulfuric acid with a concentration of 0.5M, collect the released gas with a graduated cylinder and measure the volume, so as to separate Ca...
Embodiment 2
[0037] Add 200ml of triethylenetetramine / n-decyl alcohol solution with a concentration of 0.2M into a double stirred tank with a height of 15cm and an inner diameter of 8cm, adjust the stirring speed of the liquid phase to 150rpm, and keep the temperature at 20°C through the interlayer water bath, and then pass in a solution containing 34.5% For a mixture of carbon dioxide, the inlet flow is controlled by a mass flowmeter, and the outlet flow is measured by a soap film flowmeter, which is measured every five minutes. The instantaneous absorption rate is calculated by the flow difference between the inlet and outlet, and the absorption amount is obtained by integrating the absorption rate with time. The maximum The absorption capacity is 0.070mol CO 2 .
[0038] After the absorption is completed, separate the solid phase and the liquid phase through the suction filter funnel, transfer the solid phase and the liquid phase to the flask respectively, slowly drop in dilute sulfuric...
Embodiment 3
[0040] Add 200ml of triethylenetetramine / tetrahydrofuran solution with a concentration of 0.2M to a double stirred tank with a height of 15cm and an inner diameter of 8cm, adjust the stirring speed of the liquid phase to 150rpm, and keep the temperature at 20°C through an interlayer water bath. For mixed gas, the inlet flow is controlled by a mass flowmeter, and the outlet flow is measured by a soap film flowmeter, which is measured every five minutes. The instantaneous absorption rate is calculated by the flow difference between the inlet and outlet, and the absorption capacity is obtained by integrating the absorption rate with time. The maximum absorption capacity 0.069mol CO 2 .
[0041] After the absorption is completed, separate the solid phase and the liquid phase through the suction filter funnel, transfer the solid phase and the liquid phase to the flask respectively, slowly drop in dilute sulfuric acid with a concentration of 0.5M, collect the released gas with a gra...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com