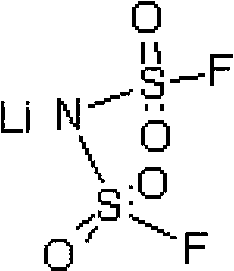
Preparation method of difluoro lithium sulfimide
A technology of lithium bisfluorosulfonyl imide and lithium bischlorosulfonyl imide, applied in the field of preparation of lithium bisfluorosulfonyl imide, can solve the problems of complex product composition, difficult separation, long process route, etc. , to achieve the effect of simple and easy-to-control process route, stable product quality and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
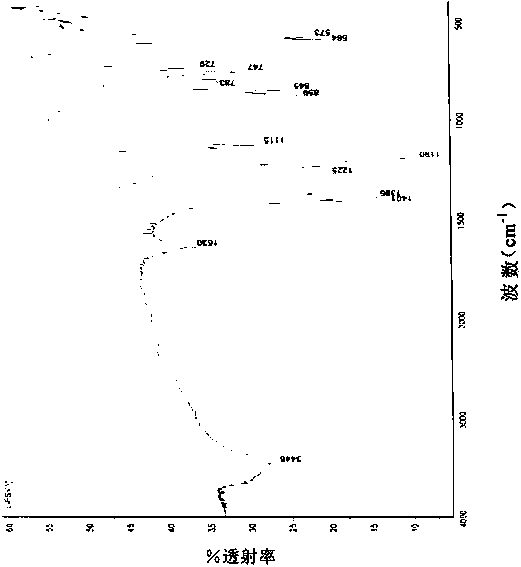
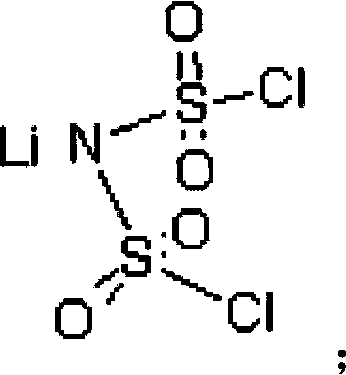
[0048] Add 220.0 g (1 mol) lithium bischlorosulfonyl imide, 220.0 g dimethyl carbonate, 52.26 g (2 mol) lithium fluoride and 0.22 g 12-crown-4-ether into a 1000 ml three-necked flask under nitrogen protection. After reacting at 40°C for 8 hours under stirring, it was cooled to room temperature, filtered, and the filtrate was evaporated to remove the solvent to obtain 149 g of white crystals, with a yield of 79.7%. The chlorine content was detected by Swiss Metrohm 848 potentiometric titrator to be 7ppm. The product has been tested by Fourier infrared, at 1401cm -1 ,1386cm -1 ,1190cm -1 ,1225cm -1 ,859cm -1 ,845cm -1 ,783cm -1 ,747cm -1 , by comparing the standard spectrum of bisfluorosulfonimide, it was determined to be the characteristic group of bisfluorosulfonimide.
Embodiment 2
[0050] Add 220.0g (1mol) lithium bischlorosulfonimide, 2200.0g diethyl carbonate, 420.0g (10mol) sodium fluoride and 22g 15-crown-5-ether into a 3000ml three-neck flask under nitrogen protection. Under stirring, react at 100°C for 24 hours, then cool to room temperature, filter, and evaporate the solvent from the filtrate to obtain 155 g of white crystals, with a yield of 82.9%. The Swiss Metrohm 848 potentiometric titrator was used to detect the chlorine content was 5ppm, and the Shimadzu AA-6300 atomic absorption instrument was used to detect the sodium ion 0.7ppm. After Fourier infrared detection, at 1401cm -1 ,1386cm -1 ,1192cm -1 ,1226cm -1 ,859cm -1 ,845cm -1 ,783cm -1 ,747cm -1 , by comparing the standard spectrum of bisfluorosulfonimide, it was determined to be the characteristic group of bisfluorosulfonimide.
Embodiment 3
[0052] Add 220.0g (1mol) lithium dichlorosulfonimide, 200.0g diethyl carbonate and 200g dipropyl carbonate, 290.0g (5mol) potassium fluoride and 22g 18-crown to a 1000ml three-necked flask under nitrogen protection -6-Ether. Under stirring, react at 130° C. for 14 hours, then cool to room temperature, filter, and evaporate the solvent from the filtrate to obtain 153 g of white crystals, with a yield of 81.8%. The Swiss Metrohm 848 potentiometric titrator was used to detect the chlorine content was 2 ppm, and the Shimadzu AA-6300 atomic absorption instrument was used to detect sodium ions and potassium ions at 0.2 ppm. After Fourier infrared detection, at 1401cm -1 ,1386cm -1 ,1190cm -1 ,1225cm -1 ,859cm -1 ,845cm -1 ,783cm -1 ,747cm -1 , by comparing the standard spectrum of bisfluorosulfonimide, it was determined to be the characteristic group of bisfluorosulfonimide.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com