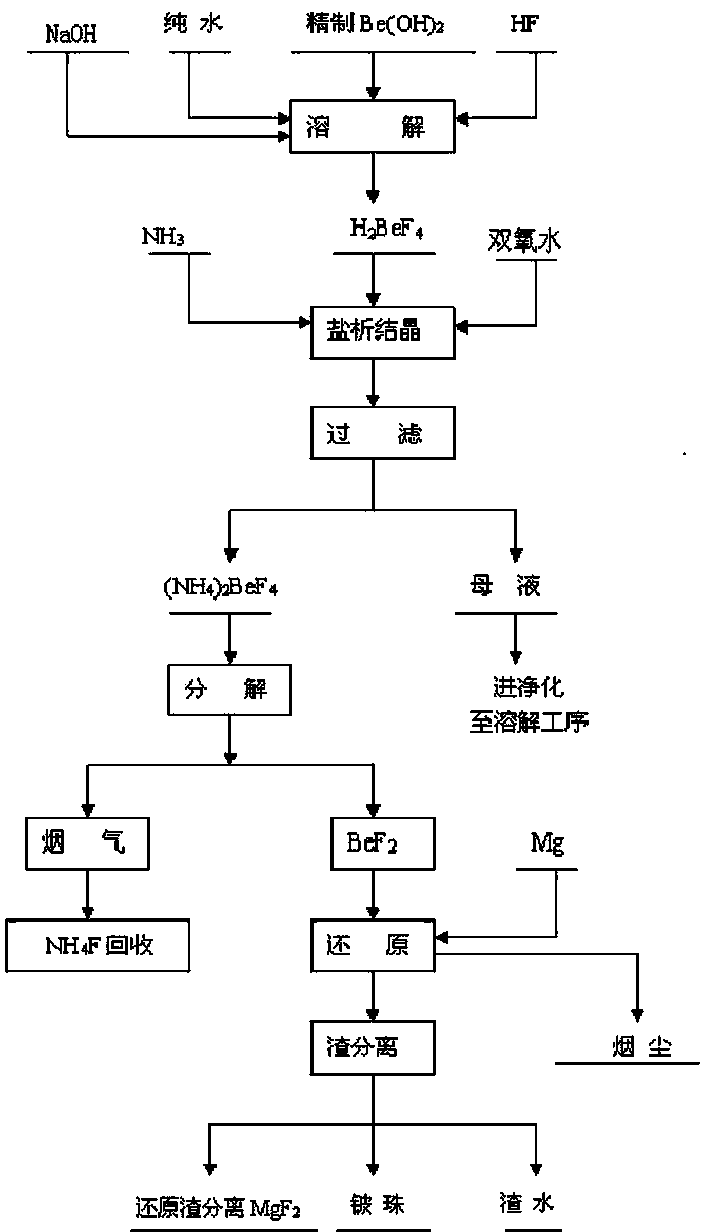
Preparation method for refined beryllium hydroxide and preparation method for reactor-quality metallic beryllium bead from refined beryllium hydroxide
A technology of beryllium hydroxide and metal, applied in the direction of beryllium oxide/hydroxide, etc., can solve the problems that cannot be realized, reference data cannot be obtained, etc., and achieve the effect of overcoming the difficulty of aluminum removal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
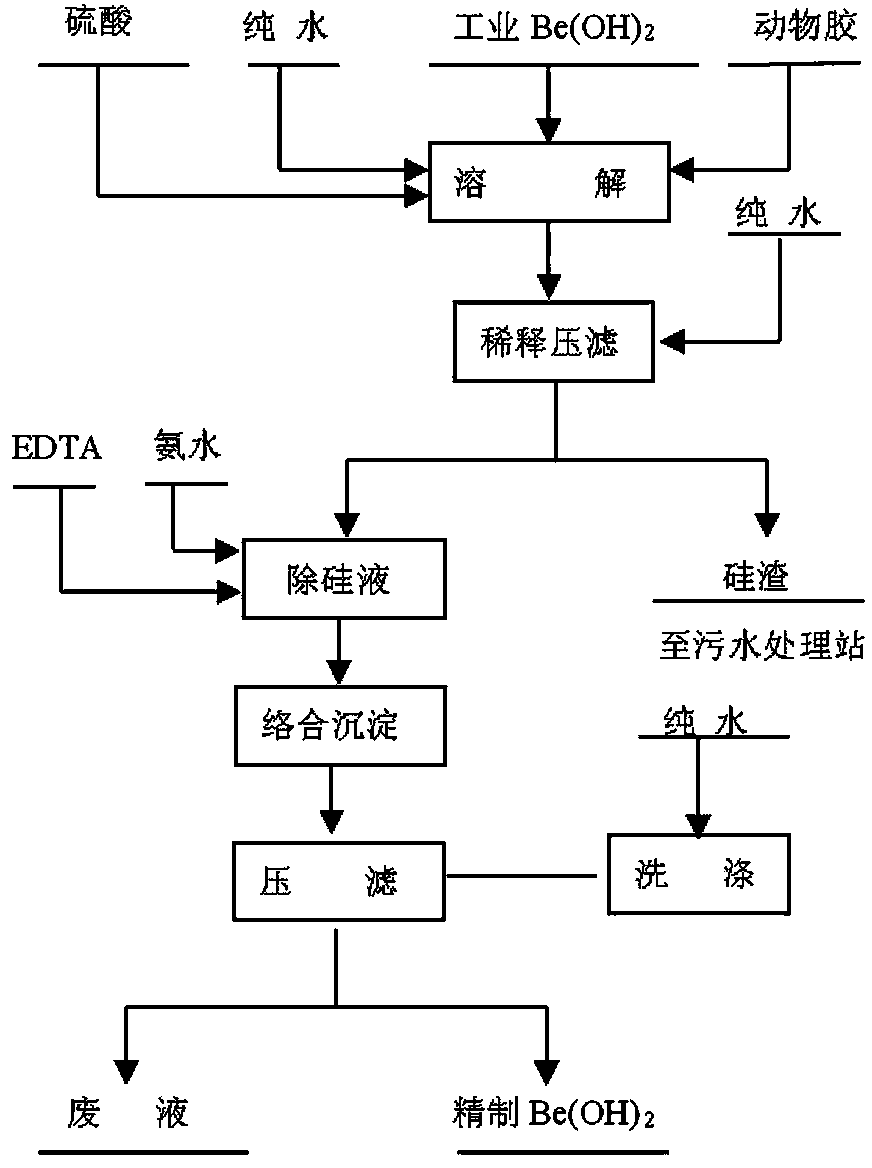
[0040] Purification of industrial beryllium hydroxide to obtain refined beryllium hydroxide preparation process, please refer to the attached figure 1 , including the following steps:
[0041] 1) Mix industrial beryllium hydroxide with water to make a slurry, press BeO:H 2 SO 4 Add sulfuric acid with a concentration of 93-98% at a weight ratio of 1:4.7, stir for 30 minutes, add beef glue at a weight ratio of BeO: animal glue of 1:0.010, heat up and evaporate to dryness, add pure water and press filter.
[0042] 2) Add pure water to the filtrate in step 1), add ammonia water to adjust the pH value to 3.5-4, raise the temperature to 70°C, add EDTA to remove impurities, stir at constant temperature for 1 hour, and pass in liquid ammonia to adjust the pH value to 5.7 After transformation, Continue to feed liquid ammonia until the pH is 8.0, stop feeding, stir for 30 minutes, and press filter to obtain refined beryllium hydroxide.
Embodiment 2
[0044] Purification of industrial beryllium hydroxide to obtain refined beryllium hydroxide preparation process, please refer to the attached figure 1 , including the following steps:
[0045] 1) Mix industrial beryllium hydroxide with water to make a slurry, press BeO:H 2 SO 4 Add sulfuric acid with a concentration of 93-98% at a weight ratio of 1:4.5. After stirring for 30 minutes, add beef glue at a weight ratio of BeO:animal glue of 1:0.012. After heating up and evaporating to dryness, add pure water and press filter.
[0046]2) Add pure water to the filtrate in step 1), add ammonia water to adjust the pH value to 3.5-4, raise the temperature to 70°C, add EDTA to remove impurities, stir at constant temperature for 1 hour, and pass in liquid ammonia to adjust the pH value to 5.7 After transformation, Continue to feed liquid ammonia until the pH is 8.0, stop feeding, stir for 30 minutes, and press filter to obtain refined beryllium hydroxide.
Embodiment 3
[0048] Purification of industrial beryllium hydroxide to obtain the preparation process of refined beryllium hydroxide, the process flow is shown in the appendix figure 1 , including the following steps:
[0049] 1) Mix industrial beryllium hydroxide with water to make a slurry, press BeO:H 2 SO 4 Add sulfuric acid with a concentration of 93-98% at a weight ratio of 1:5. After stirring for 30 minutes, add beef gum at a weight ratio of BeO: animal glue of 1:0.008. After heating up and evaporating to dryness, add pure water and press filter.
[0050] 2) Add pure water to the filtrate in step 1), add ammonia water to adjust the pH value to 3.5-4, raise the temperature to 70°C, add EDTA to remove impurities, stir at constant temperature for 1 hour, and pass in liquid ammonia to adjust the pH value to 5.7 After transformation, Continue to feed liquid ammonia until the pH is 8.0, stop feeding, stir for 30 minutes, and press filter to obtain refined beryllium hydroxide.
[0051] T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com