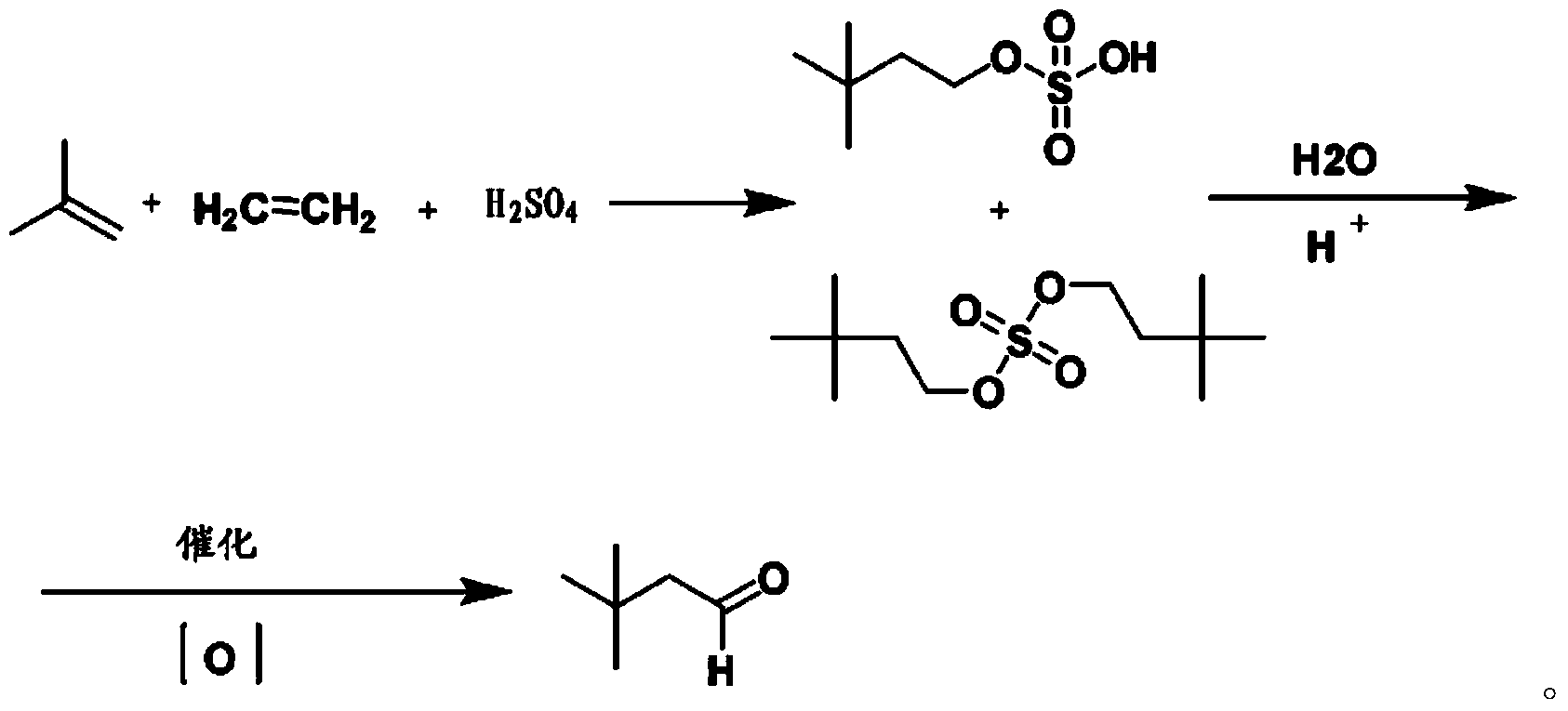
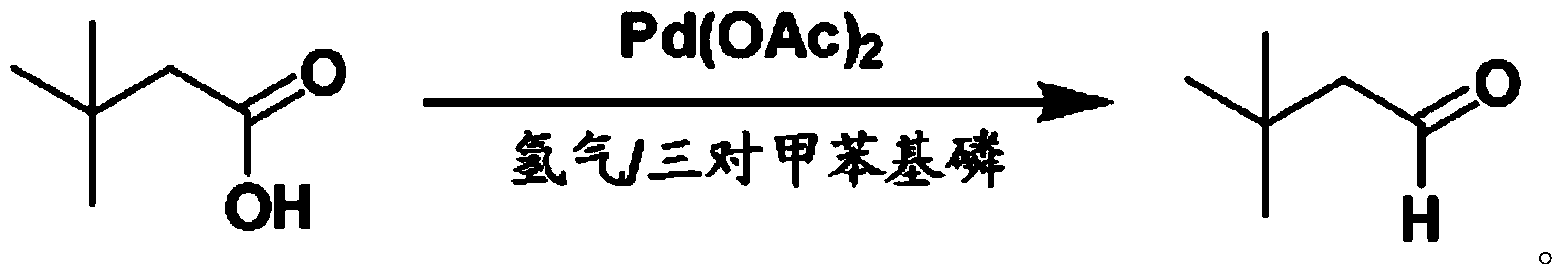
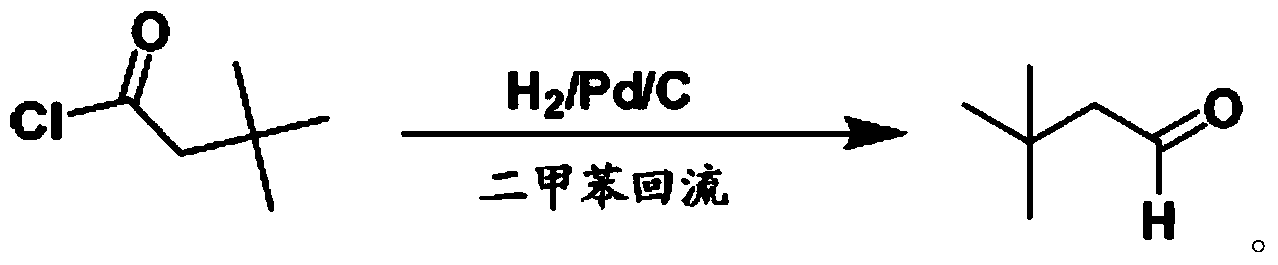
Preparation method for 3,3-dimethylbutyraldehyde
A technology of dimethyl butyraldehyde and dimethyl butanol, which is applied in the field of preparation of 3,3-dimethyl butyraldehyde, can solve the problems of violent exothermic reaction, difficult control of large-scale production, low product purity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Add 51g (0.5mol) of 3,3-dimethylbutanol and 2,2,6,6-tetramethyl-1-piperidinyl oxygen radical and potassium bromide compound salt 9.8 into a 2000ml four-necked flask g (0.05mol), cooled to -10°C, and began to add 10% NaClO dropwise 2 Aqueous solution 1357ml (containing 1.5mol NaClO 2 ), keep the temperature between 0~-5°C during the dropping process, continue to stir after the dropping, slowly raise the temperature to 25°C for oxidation reaction, until the content of 3,3-dimethylbutanol is ≤1% when detected by GC Reaction finishes; after reaction finishes, add dichloromethane 300ml to extraction in reaction solution, then organic layer is washed with water, concentrated, vacuum distillation, obtains 40g of target product, and the content of 3,3-dimethylbutyraldehyde in target product is 98.5%, the yield is 80%.
Embodiment 2
[0039] Add 102g (1.0mol) of 3,3-dimethylbutanol, 500ml of THF and the compound of 2,2,6,6-tetramethyl-1-piperidinyloxy radical and potassium bromide into a 5000ml four-necked flask Salt 19.6g (0.1mol), cooled to -10°C, began to drop 10% NaClO by mass 2 2714ml aqueous solution (containing 3molNaClO 2 ), keep the temperature between 0~-5°C during the dropping process, continue to stir after the dropping, slowly raise the temperature to 25°C for oxidation reaction, until the content of 3,3-dimethylbutanol is ≤1% when detected by GC Reaction finishes; after reaction finishes, add dichloromethane 600ml to extraction in reaction liquid, then organic layer is washed with water, concentrates, decompression distillation, obtains 85g of target product, and the content of 3,3-dimethylbutyraldehyde in target product is 98.5%, the yield is 85%.
Embodiment 3
[0041] Add 102g (1.0mol) of 3,3-dimethylbutanol, 2,2,6,6-tetramethyl-1-piperidinyl oxygen radical and potassium bromide compound salt 19.6 into a 5000ml four-necked flask g (0.1mol) and 10g of butylammonium bromide, cooled to -10°C, and began to add dropwise NaClO with a mass percentage of 10%. 2 2714ml aqueous solution (containing 3molNaClO 2 ), keep the temperature between 0~-5°C during the dropping process, continue to stir after the dropping, slowly raise the temperature to 25°C for oxidation reaction, until the content of 3,3-dimethylbutanol is ≤1% when detected by GC The reaction is over; after the reaction is over, add 600ml of dichloromethane to the reaction solution for extraction, then wash the organic layer with water, concentrate, and distill under reduced pressure to obtain 87g of the target product, and the content of 3,3-dimethylbutyraldehyde in the target product is 98.6%, the yield is 87%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com