Method for synthesizing cyclic carbonate by catalysis of amino functional ionic liquid
A technology of amino-functionalization and cyclic carbonate, applied in the direction of organic chemistry, can solve the problems of harsh reaction conditions, low catalyst activity, and difficulty in carbon dioxide activation, and achieve high reactivity, good industrial application prospects, and mild reaction conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
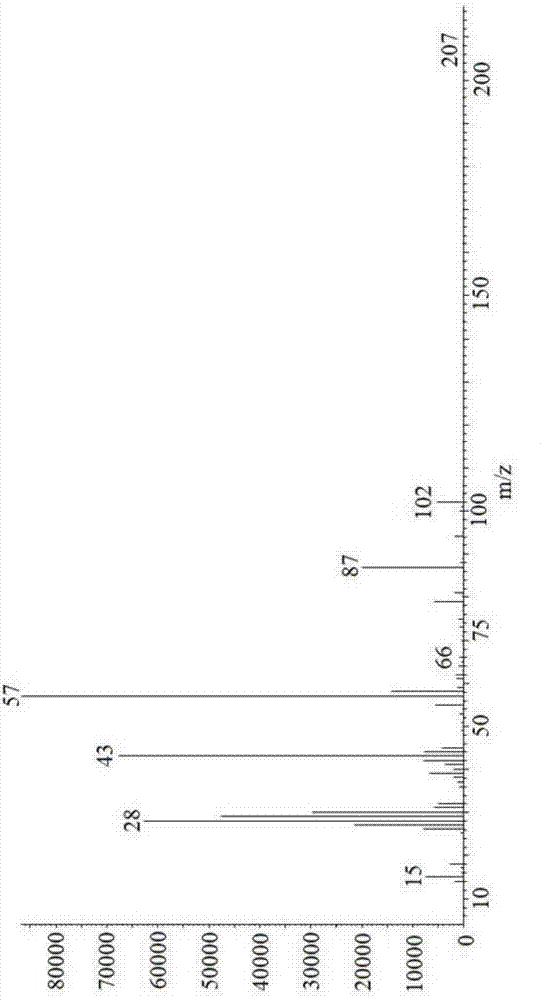
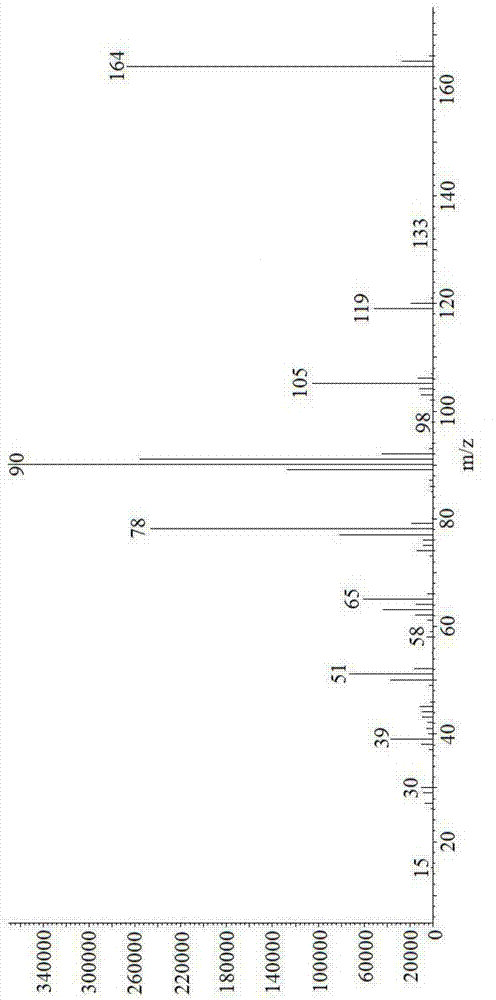
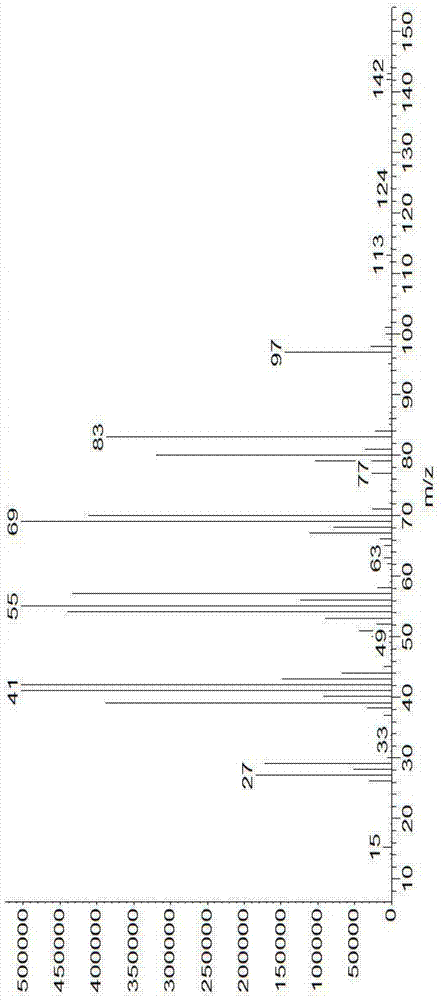
Image
Examples
specific Embodiment approach 1
[0018] Specific embodiment 1: This embodiment is a method for catalytically synthesizing a cyclic carbonate with an amino-functionalized ionic liquid, which is specifically completed according to the following steps:
[0019] Add amino-functionalized ionic liquid to the autoclave, then add epoxy compound, the molar ratio of described amino-functionalized ionic liquid and epoxy compound is (1~50):1000, airtight autoclave, under normal pressure and temperature A constant temperature reaction in an oil bath at 25-200°C for 0.5h-24.0h, and then cooling to room temperature in an ice-water mixture to obtain a reaction product, which is distilled under reduced pressure using a rotary evaporator to obtain a cyclic carbonate.
[0020] The chemical formula of the amino functionalized ionic liquid described in this embodiment is as stated in Wherein X is Cl, Br or I, n=1, 2, 3 or 4; the described Wherein X is Cl, Br or I, n=1, 2, 3 or 4; the described In which X is Cl, Br or I, ...
specific Embodiment approach 2
[0026] Embodiment 2: The difference between this embodiment and Embodiment 1 is that the molar ratio of the amino functionalized ionic liquid to the epoxy compound is (5-50):1000. Others are the same as the first embodiment.
specific Embodiment approach 3
[0027] Embodiment 3: This embodiment differs from Embodiment 1 or Embodiment 2 in that the molar ratio of the amino-functionalized ionic liquid to the epoxy compound is 10:1000. Others are the same as those in Embodiment 1 or 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com