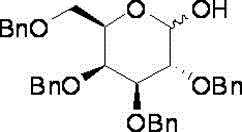
A method for preparing 2,3,4,6-tetra-oxo-benzyl-d-galactopyranose
A kind of technology of galactopyranose and benzothiazolylthiotetrabenzyl half, applied in the field of preparation of 2,3,4,6-tetra-oxo-benzyl-D-galactopyranose, can solve the problem of yield Low cost, high cost, etc., to achieve the effect of easy to obtain raw materials, simple operation, saving operating costs and materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 15
[0025] Example 15.5:2:1:1.1
[0026] a) Add 5.5mol acetic anhydride and 2mol zinc chloride at room temperature, add 1mol galactose in 10 batches at 10-15°C, after the reaction is complete, add 1.1mol 2-mercaptobenzothiazole, heat to 50-60°C; after post-treatment 0.87 mol of benzothiazolylthioacetylgalactose was obtained.
[0027] b) Add 3.48 mol of potassium hydroxide and 0.87 mol of benzothiazolyl thioacetylgalactose to 8.7 mol of benzyl chloride and heat to reflux. After the reaction is completed, the temperature is lowered, and 0.79 mol of benzothiazolyl thiogalactose is obtained after post-treatment. Benzylgalactose.
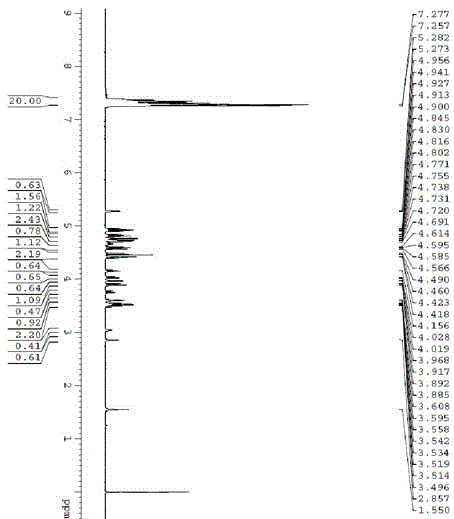
[0028] c) Add 0.79 mol of benzothiazolylthiotetrabenzylgalactose to 4 mol of acetone to dissolve, add 2 mol of water, stir at room temperature, add 0.87 mol of N-bromosuccinimide, stir for 0.5 hours, after the reaction is completed The treatment yielded 0.71mol 2,3,4,6-tetra-oxo-benzyl-D-galactopyranose with a yield of 71%.
[0029] The post-treatment in ...
Embodiment 26
[0032] Example 26:2:1:1.2
[0033] a) Add 6mol acetic anhydride and 2mol zinc chloride at room temperature, add 1mol galactose in 10 batches at 10-15°C, add 1.2mol 2-mercaptobenzothiazole after the reaction is completed, and heat to 50-60°C; 0.88 mol benzothiazolylthioacetylgalactose.
[0034] b) Add 3.6 mol of potassium hydroxide and 0.88 mol of benzothiazolyl thioacetylgalactose to 9.2 mol of benzyl chloride and heat to reflux. After the reaction is completed, the temperature is lowered, and 0.81 mol of benzothiazolyl thiogalactose is obtained after post-treatment. Benzylgalactose.
[0035] c) Add 0.81 mol of benzothiazolylthiotetrabenzylgalactose to 4 mol of acetone to dissolve, add 2 mol of water, stir, add 0.94 mol of N-bromosuccinimide at room temperature, stir for 0.5 hours, after the reaction is completed The treatment yielded 0.72mol 2,3,4,6-tetra-oxo-benzyl-D-galactopyranose with a yield of 72%.
[0036] The post-treatment in step a is to add dropwise 10mol of wat...
Embodiment 36
[0039] Example 36.5:2:1:1.2
[0040] a) Add 65mol acetic anhydride and 20mol aluminum chloride at room temperature, add 10mol galactose in 10 batches at 10-15°C, add 12mol 2-mercaptobenzothiazole after the reaction is completed, and heat to 50-60°C; after post-treatment, 8.9 mol benzothiazolylthioacetylgalactose.
[0041] b) Add 44.5 mol of potassium hydroxide and 8.9 mol of benzothiazolyl thioacetylgalactose to 106.8 mol of benzyl chloride and heat to reflux. After the reaction is completed, the temperature is lowered, and 8.25 mol of benzothiazolyl thiogalactose is obtained after post-treatment. Benzylgalactose.
[0042]c) Add 8.25 mol of benzothiazolylthiotetrabenzylgalactose to 40 mol of acetone to dissolve, add 20 mol of water, stir at room temperature, add 9.6 mol of N-bromosuccinimide, stir for 0.5 hours, after the reaction is completed The treatment yielded 7.33mol2,3,4,6-tetra-oxo-benzyl-D-galactopyranose with a yield of 73.3%.
[0043] The post-treatment in step a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com