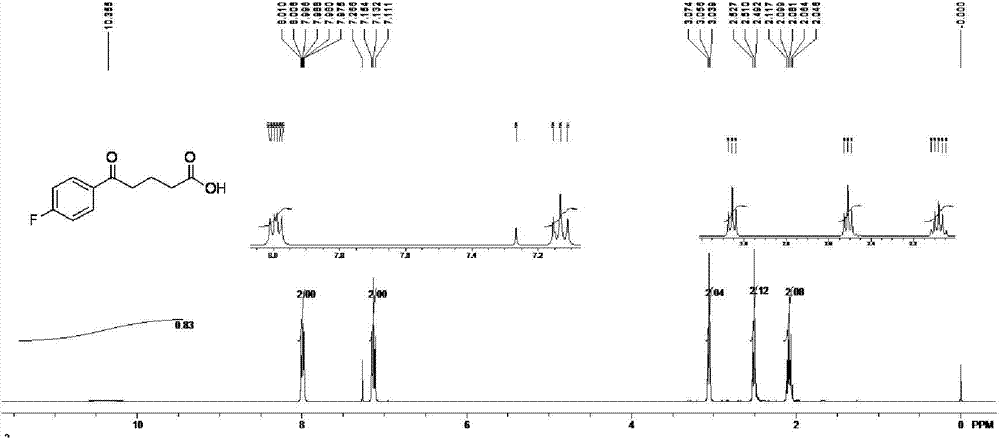
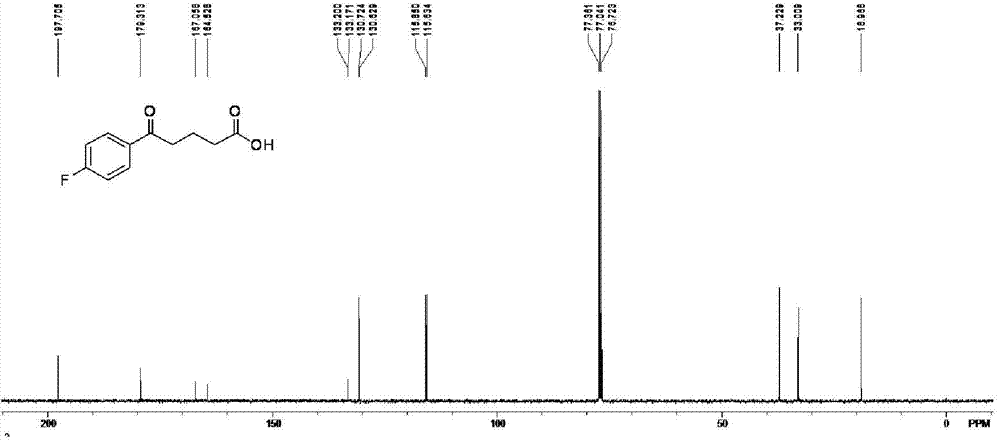
Method for preparing 4-(4-fluorobenzoyl) butyric acid
A technology of fluorobenzoyl and fluorobenzene, applied in the field of preparation of 4-butyric acid, can solve the problems of high price, high toxicity of fluorobenzene, troublesome handling, etc., and achieves the advantages of low production cost, improved yield and reduced usage. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~11
[0033] Treatment of aluminum trichloride: anhydrous aluminum trichloride (purchased from Sinopharm Chemical Reagent Co., Ltd., product number 10000862). Aluminum trichloride was ground into powder in a mortar, sieved with a 40-mesh screen to obtain fine particles of aluminum trichloride, and then the fine particles were baked at 110°C for 1 hour until a large amount of white smoke came out, and then the fine particles were dried under nitrogen protection. Allow to cool down to room temperature.
[0034] Preparation of 4-(4-fluorobenzoyl)butyric acid: under nitrogen protection, add processed aluminum trichloride, fluorobenzene and organic solvent 100mL in a 500mL three-necked bottle according to the raw material ratio in Table 1, mix Stir evenly, put the three-necked bottle under an ice bath, and add glutaric anhydride solution dropwise to it, wherein, the solvent used for the glutaric anhydride solution is the same as that used for dissolving fluorobenzene, and the dosage is 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com