Amphiphilic C-6-(4-(methyl amino)-1,2,3-triazole)deoxidized inulin derivatives and preparation and application thereof
A technology of deoxyinulin and methyl amino, which is applied in the field of daily chemicals, can solve the problems of few renewable resources, etc., and achieve the effects of low synthesis cost, expanded application range, and enhanced solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
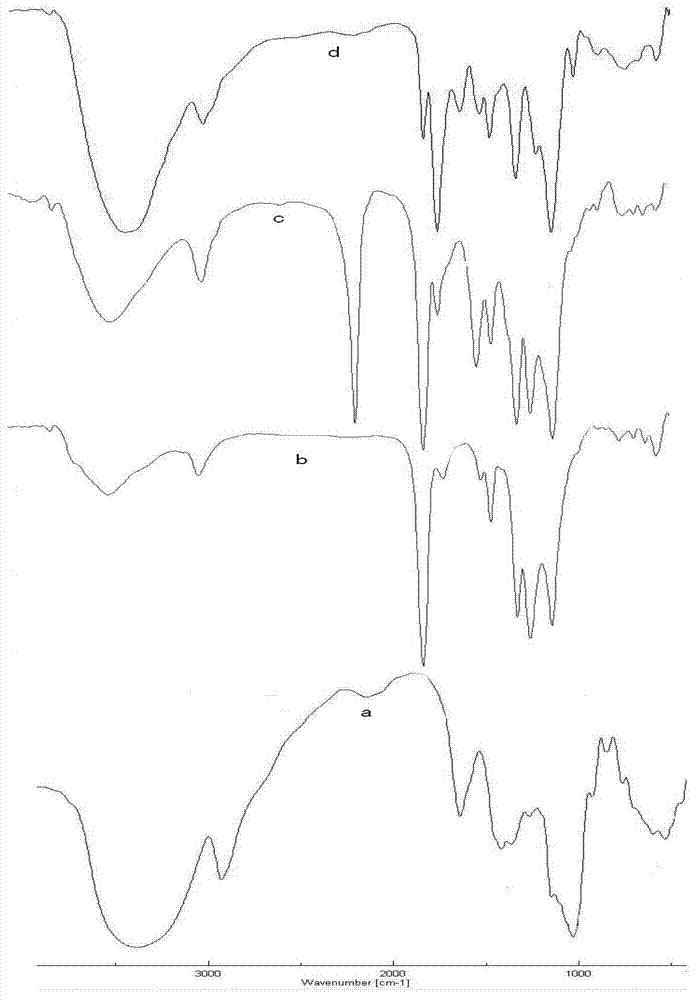
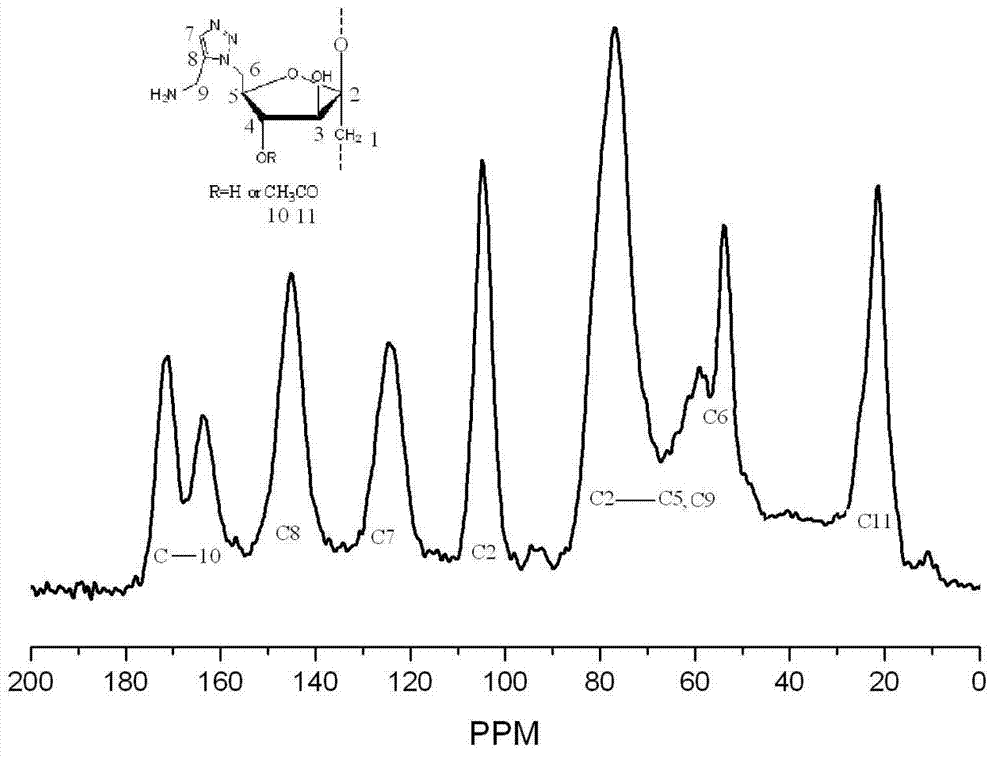
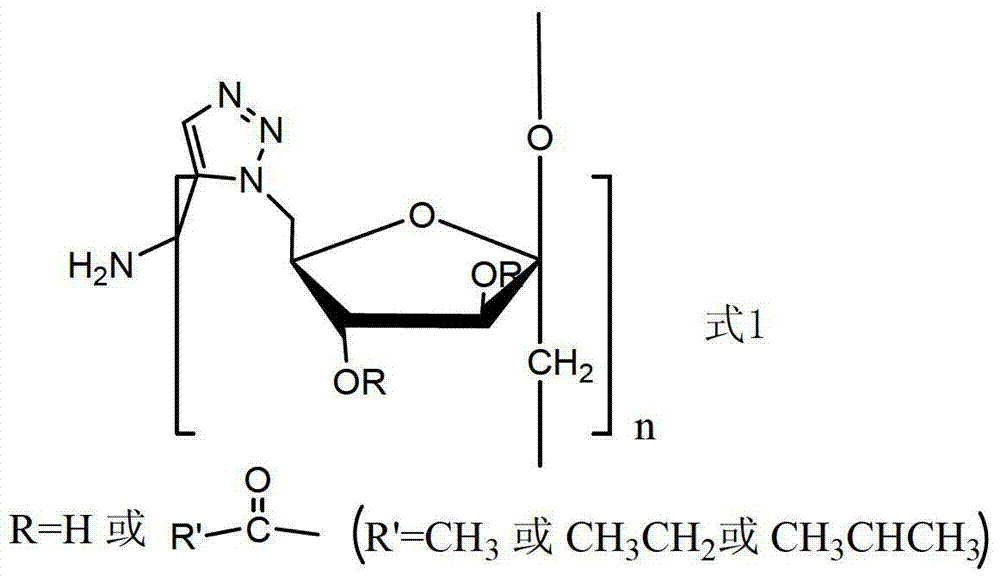
[0026] The amphiphilic C-6-(4-(methylamino)-1,2,3-triazole) deoxyinulin derivative is a compound represented by formula (1).
[0027]
[0028]
[0029] This embodiment synthesizes the target compound according to the above synthetic route (1):
[0030] 1) Synthesis of Compound 1: Inulin was dried overnight at 100°C under vacuum. Take 1.62g of inulin and add it to 50mL of purified nitrogen-nitrogen dimethylformamide under the protection of nitrogen, raise the temperature until the inulin is completely dissolved, and add 3.5g of N-bromosuccinyl when the temperature of the reaction solution drops to room temperature imine (NBS) to the above reaction solution. Weigh 5.2g of triphenylphosphine and dissolve it in 30mL of nitrogen nitrogen dimethylformamide, and add it dropwise to the above reaction solution at room temperature after dissolving. After reacting at room temperature for 30 minutes, the temperature of the reaction system was raised to 70° C., and reacted at this ...
Embodiment 2
[0037] The difference from Example 1 is:
[0038] 1) Synthesis of Compound 1: Inulin was dried overnight at 100°C under vacuum. Take 1.62g of inulin and add it to 50mL of purified nitrogen-nitrogen dimethylformamide under the protection of nitrogen, raise the temperature until the inulin is completely dissolved, and add 3.5g of N-bromosuccinyl when the temperature of the reaction solution drops to room temperature imine (NBS) to the above reaction solution. Weigh 5.2 g of triphenylphosphine and dissolve it in 30 mL of nitrogen-nitrogen dimethylformamide. This solution was added dropwise to the reaction solution at room temperature. After the reaction solution was reacted at room temperature for 30 minutes, the temperature of the reaction system was raised to 70°C. After the reaction was carried out at 70°C for 3 hours, the reaction solution was poured into 250mL acetone, and a precipitate was precipitated. After the precipitate was suction-filtered and washed with acetone, i...
Embodiment 3
[0042] The difference from Example 1 is:
[0043] 1) Synthesis of Compound 1: Inulin was dried overnight at 100°C under vacuum. Take 1.62g of inulin and add it to 50mL of purified nitrogen-nitrogen dimethylformamide under the protection of nitrogen, raise the temperature until the inulin is completely dissolved, and add 3.5g of N-bromosuccinyl when the temperature of the reaction solution drops to room temperature imine (NBS) to the above reaction solution. Weigh 5.2 g of triphenylphosphine and dissolve it in 30 mL of nitrogen-nitrogen dimethylformamide. This solution was added dropwise to the reaction solution at room temperature. After the reaction solution was reacted at room temperature for 30 minutes, the temperature of the reaction system was raised to 70°C. After the reaction was carried out at 70°C for 3 hours, the reaction solution was poured into 250mL acetone, and a precipitate was precipitated. After the precipitate was suction-filtered and washed with acetone, i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com