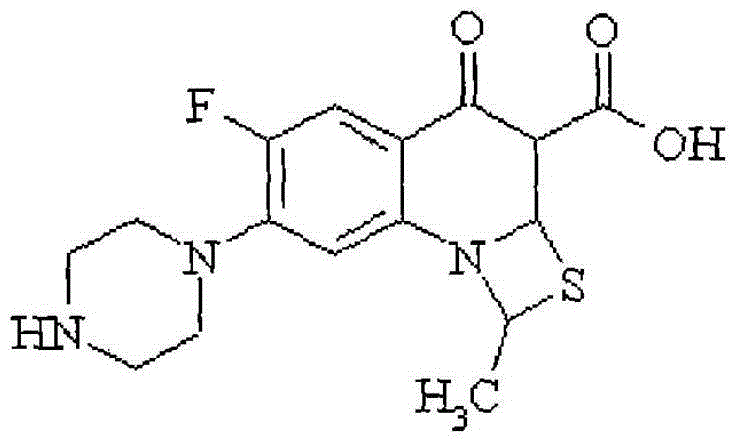
A kind of Ulifloxacin eye drops and preparation method thereof
A technology of ulifloxacin and eye drops, which is applied in the direction of medical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problems of side effects and drug resistance, and achieve low toxic and side effects The effect of strong activity and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Ulifloxacin eye drops contain 3mg of ulifloxacin, 40mg of polyvinylpyrrolidone, and 0.3mg of ethylparaben per milliliter.
[0064] prescription:
[0065] Ulifloxacin 30g
[0066] Polyvinylpyrrolidone (Povidone PVP) 400g
[0067] Ethylparaben 3g
[0068] Acetic acid 20mL
[0069] Appropriate amount of glycerin
[0070] Appropriate amount of acetic acid buffer
[0071] Add water for injection to 10L.
[0072] Preparation Process:
[0073] Dissolve 30g of ulifloxacin in 2L of water for injection containing 20mL of acetic acid; dissolve 400g of polyvinylpyrrolidone, 3g of ethylparaben, and 300ml of glycerin in 5L of water for injection, and dissolve the two liquids; Add acetic acid buffer solution, add water for injection to 10L, check the content of the semi-finished product, adjust the pH value to 5.0, sterilize by flowing steam at 100°C for 30 minutes, cool to room temperature, filter with 0.22um microporous membrane, aseptically dispense and package Instantly. ...
Embodiment 2
[0076] Ulifloxacin eye drops, the finished product contains 1mg of Ulifloxacin, 40mg of polyvinylpyrrolidone, and 0.4mg of ethylparaben per milliliter.
[0077] prescription:
[0078] Ulifloxacin 5g
[0079] Polyvinylpyrrolidone 200g
[0080] Ethylparaben 2g
[0081] Acetic acid 10mL
[0082] Appropriate amount of glycerin
[0083] Appropriate amount of acetate buffer
[0084] Add water for injection to 5L.
[0085] Preparation Process:
[0086] Dissolve 5 g of ulifloxacin in 1 L of water for injection containing 10 mL of acetic acid; dissolve 200 g of polyvinylpyrrolidone, 1.5 g of ethylparaben, and 150 ml of glycerin in 3 L of water for injection, and heat to dissolve; Add acetic acid buffer solution to the filter, add water for injection to about 5L, adjust the pH value to 6.5±0.5, the content of the semi-finished product is qualified, sterilize it by circulating steam at 100°C for 30 minutes, cool to room temperature, filter with a 0.22um microporous membrane, and m...
Embodiment 3
[0088] Ulifloxacin eye drops, the finished product contains 1mg of Ulifloxacin, 40mg of polyvinylpyrrolidone, and 0.3mg of ethylparaben per milliliter.
[0089] prescription:
[0090] Ulifloxacin 10g
[0091] Polyvinylpyrrolidone 400g
[0092] Ethylparaben 3g
[0093] Acetic acid 20mL
[0094] Appropriate amount of glycerin
[0095] Appropriate amount of acetic acid buffer
[0096] Add water for injection to 10L.
[0097] Preparation Process:
[0098] Dissolve 10g of ulifloxacin in 2L of water for injection containing 20mL of acetic acid; dissolve 400g of polyvinylpyrrolidone, 3g of ethylparaben, and 300ml of glycerin in 5L of water for injection, heat to dissolve; combine the two liquids, filter, and filter Add acetic acid buffer solution, add water for injection to make up to 10L, adjust the pH value to 5.5, sterilize by flowing steam at 100°C for 30 minutes, cool to room temperature, check the content of the semi-finished product, filter it with a 0.22um microporous ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com