3, 4-difluorobenzonitrile preparation method
A technology of difluorobenzonitrile and difluorobenzene, which is applied in the field of preparation of 3,4-difluorobenzonitrile, can solve the problems of serious pollution and high cost, and achieve the effects of high product purity, low cost and easy industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
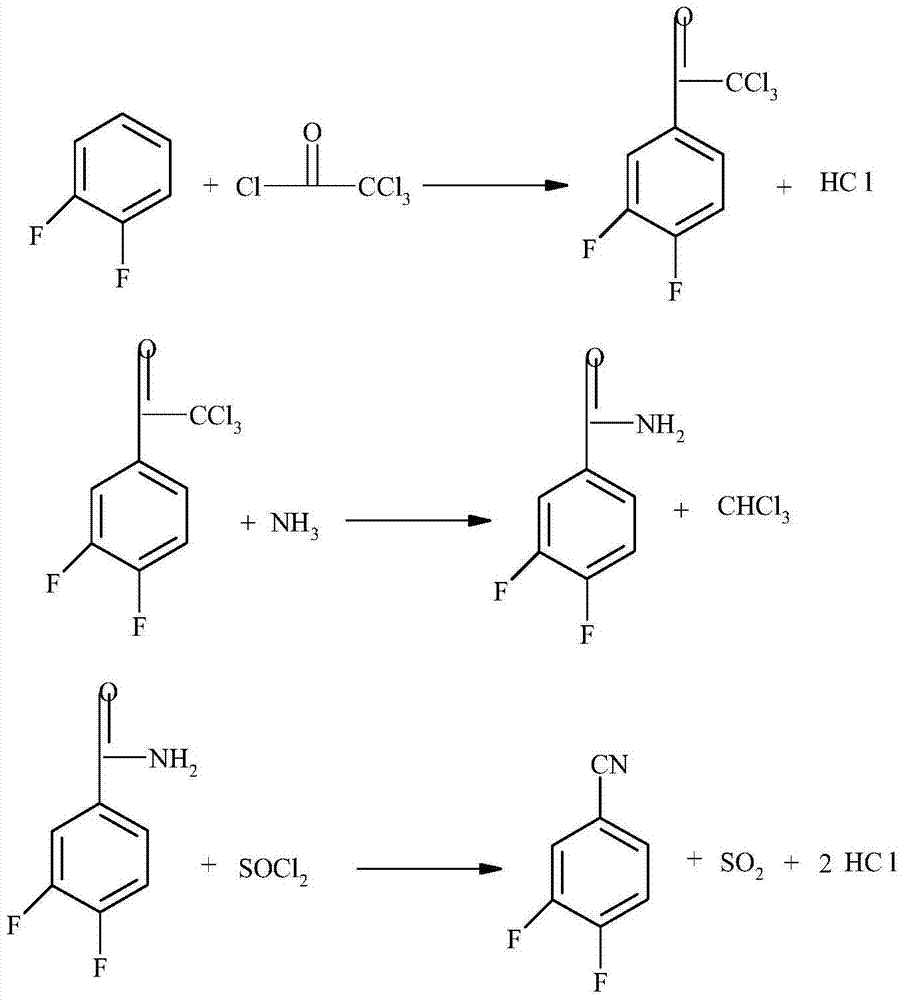
[0030] Add 228.4kg (2.0kmol) of 1,2-difluorobenzene, 382.4kg (2.2kmol) of trichloroacetyl chloride and 1600kg of dichloroethane into the dry reactor, stir to dissolve, add anhydrous zinc chloride 220 kg, a large amount of gas escaped, and the reaction temperature was controlled at 25-30°C under an ice-water bath. After the raw materials were completely reacted, the reaction liquid was poured into ice water under stirring, and 2093 kg containing 3,4-difluoro -(α,α,α-Trichloroacetyl)benzene 493.0 kg (1.9 kilomole) dichloroethane solution, yield 95%.
[0031] In another reactor, add 2093 kg of 3,4-difluoro-(α, α, α-trichloroacetyl) benzene 493.0 kg (1.9 kilomole) of dichloroethane solution, stir ammonia gas and control The reaction temperature is 20-30°C, and a white solid precipitates out. After the raw materials are completely reacted, filter, wash, and dry to obtain 289.6 kg (1.843 kmol) of 3,4-difluorobenzamide, with a yield of 97%
[0032] Add 289.6 kilograms (1.843 kilomol...
Embodiment 2
[0035] Add 228.4 kilograms (2.0 kilomoles) of 1,2-difluorobenzene, 382.4 kilograms (2.2 kilomoles) of trichloroacetyl chloride and 1600 kilograms of dichloroethane into the dry reactor, stir to dissolve, add anhydrous aluminum chloride 240 kg, a large amount of gas escaped, and the reaction temperature was controlled at 25-30°C under an ice-water bath. After the raw materials were completely reacted, the reaction liquid was poured into ice water under stirring, and 2093 kg of 3,4-difluoro- (α,α,α-Trichloroacetyl)benzene 493.0 kg (1.9 kilomole) dichloroethane solution, yield 95%.
[0036] Add 2093 kg of dichloroethane solution containing 493.0 kg (1.9 kilomole) of 3,4-difluoro-(α, α, α-trichloroacetyl)benzene in another reactor, and pass ammonia gas under stirring. Control the reaction temperature at 20-30°C, and a white solid precipitates out. After the reaction of the raw materials is complete, filter, wash, and dry to obtain 292.6 kg (1.862 kmol) of 3,4-difluorobenzamide, wi...
Embodiment 3
[0040] Add 228.4kg (2.0kmol) of 1,2-difluorobenzene, 382.4kg (2.2kmol) of trichloroacetyl chloride and 1600kg dichloroethane into the dry reactor, stir to dissolve, add anhydrous aluminum chloride 240 kg, a large amount of gas escapes, and the reaction temperature is controlled in an ice-water bath at 25-30°C. After the raw materials are completely reacted, the reaction solution is poured into ice water under stirring, and separated and washed to obtain 3,4-difluoro-( α, α, α-trichloroacetyl) benzene 493.0 kg (1.9 kilomoles), yield 95%.
[0041]Add 493.0 kg (1.9 kilomoles) of 3,4-difluoro-(α, α, α-trichloroacetyl)benzene and 1600 kg of chlorobenzene into another reactor, pass ammonia under stirring and control the reaction temperature to 20- At 30°C, a white solid precipitated out. After the raw materials were completely reacted, they were filtered, washed, and dried to obtain 292.6 kg (1.862 kmol) of 3,4-difluorobenzamide, with a yield of 98%.
[0042] Add 292.6 kilograms (1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com