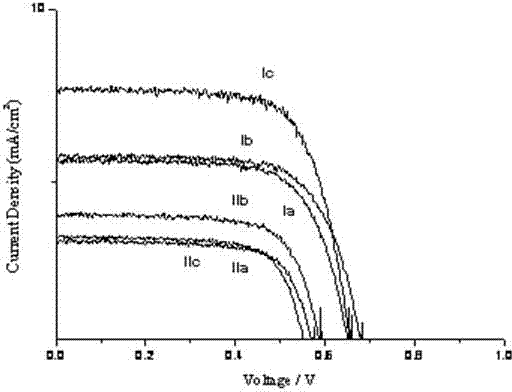
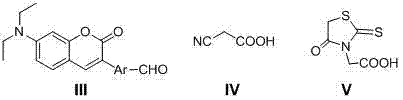
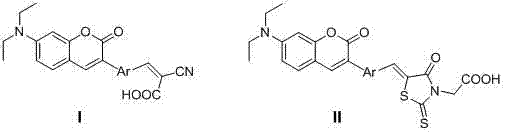
Synthesis and application of diethylamino coumarin dye sensitizer
A technology of coumarins and diethylamino, which is applied in the field of synthesis of diethylaminocoumarin dye sensitizers, can solve the problems that there are no literature reports on the application of solar cells, achieve good photoelectric conversion efficiency, and prepare methods simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036]
[0037] 7-Diethylamino-3-p-formylphenylcoumarin IIIa Synthesis
[0038] 3-bromo-7-diethylaminocoumarin VI (1.18 g, 4 mmol ), 4-formylphenylboronic acid VII (0.75 g, 5 mmol ) and tetrakistriphenylphosphine palladium (462 mg, 0.4 mmol ) were dissolved in dry THF 40 ml, and aqueous sodium carbonate solution (2.8 ml, 2 M ) was added. N 2 Refluxed for 8 h under protection, cooled to room temperature, and spin-dried the solvent to obtain a yellow solid (690 mg) with a yield of 54% after separation on a PE:EA=5:1 column. m.p.: 145-147 oC; 1 H NMR δ (500 MHz, CDCl 3 ): 10.03 (s, 1H, C H O), 7.93-7.89 (m, 4H, Ar-H), 7.82 (s, 1H, Ar-H), 7.35 (d, J = 8.82 Hz, 1H, Ar-H), 6.63 (dd, J = 2.38, 8.88 Hz, 1H, Ar-H), 6.53 (d, J = 2.31 Hz, 1H, Ar-H), 3.45 (q, J = 7.12 Hz, 4H, N(C H 2 CH 3 ) 2 ), 1.23 (t, J = 7.12 Hz, 6H, N(CH 2 C H 3 ) 2); HREIMS m / z 344.1156 [M+Na] + , calcd for C 20 h 19 NO 3 Na: 344.3596.
[0039] 2-cyano-3-(4-(7-(diethylamino)-cou...
Embodiment 2
[0042]
[0043] 5-(4-(7-(Diethylamino)-coumarin-3-yl)phenyl)-3-carboxymethylrhodanine IIa Synthesis
[0044] 7-Diethylamino-3-p-formylphenylcoumarin IIIa (0.032 g, 0.1 mmol) and rhodanine acetate V (0.038 g, 0.2 mmol) was dissolved in 2 ml acetonitrile and 1 ml THF, piperidine (0.001 ml, 0.01 mmol) was added, N 2 After reflux for 2 h under protection, the solvent was spin-dried, HAc:MeOH:CH 2 Cl 2 =1:5:100 column separation to obtain a red solid (33 mg), yield 67%. m.p.: > 300 oC; 1 H NMR δ (500 MHz, DMSO- d ): 8.27 (s, 1H, Ar-H), 7.96 (t, J = 8.48 Hz, 2H, Ar-H), 7.92 (s, 1H, Ar-H), 7.72 (d, J = 8.49 Hz, 2H, Ar-H), 7.54 (d, J = 8.92 Hz, 1H, Ar-H), 6.77 (dd, J = 2.26, 8.99 Hz, 1H, Ar-H), 6.59 (d, J = 2.15 Hz, 1H, Ar-H), 4.75 (s, 2H, C H 2 ), 3.47 (q, J = 7.03 Hz, 4H, N(C H 2 CH 3 ) 2 ), 1.16 (t, J = 6.98 Hz, 6H, N(CH 2 C H 3 ) 2 ); HREIMS m / z 493.0899 [M-H] - , calcd for C 25 h 21 N 2 o 5 S 2 : 493.5747.
Embodiment 3
[0046]
[0047] 4'-(7-(Diethylamino)-coumarin-3-yl)-[1,1'-biphenyl]-4-aldehyde IIIb Synthesis
[0048] 7-Diethylamino-3-p-bromophenylcoumarin VIII (1.4 g, 3.8 mmol), 4-formylphenylboronic acid VII (0.69 g , 4.6 mmol), tetrakistriphenylphosphine palladium (439 mg , 0.38 mmol) were dissolved in dry THF 40ml, and aqueous sodium carbonate solution (2.8 ml, 2 M) was added, N 2 Reflux for 8 h under protection, cool to room temperature, and spin dry the solvent. PE: EA = 5:1 After column separation, a yellow solid (500 mg, 1.26 mmol) was obtained with a yield of 33%. m.p.: 240-242 oC; 1 H NMR δ (500 MHz, CDCl 3 ): 10.08 (s, 1H, C H O), 7.98 (d, J = 8.09 Hz, 2H, Ar-H), 7.86-7.79 (m, 5H, Ar-H), 7.70 (d, J = 8.23 Hz, 2H, Ar-H), 7.35 (d, J = 8.77 Hz, 1H, Ar-H), 6.63 (dd, J = 2.02, 8.82 Hz, 1H, Ar-H), 6.56 (s, 1H, Ar-H), 3.45 (q, J = 7.08 Hz, 4H, N(C H 2 CH 3 ) 2 ), 1.24 (t, J = 7.06 Hz, 6H, N(CH 2 C H 3 ) 2 ); HREIMS m / z 420.1515 [M+Na] + , calcd for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com