A method for stepwise recovery of valuable metals from zinc-manganese-cobalt-rich slag with multiple controls
A zinc-manganese-cobalt-rich slag, multi-control technology, applied in the improvement of process efficiency, photography process, instruments, etc., can solve the problems of high equipment requirements, complex operation, long process, etc., achieve low equipment requirements, simple operation, avoidance of The effect of pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
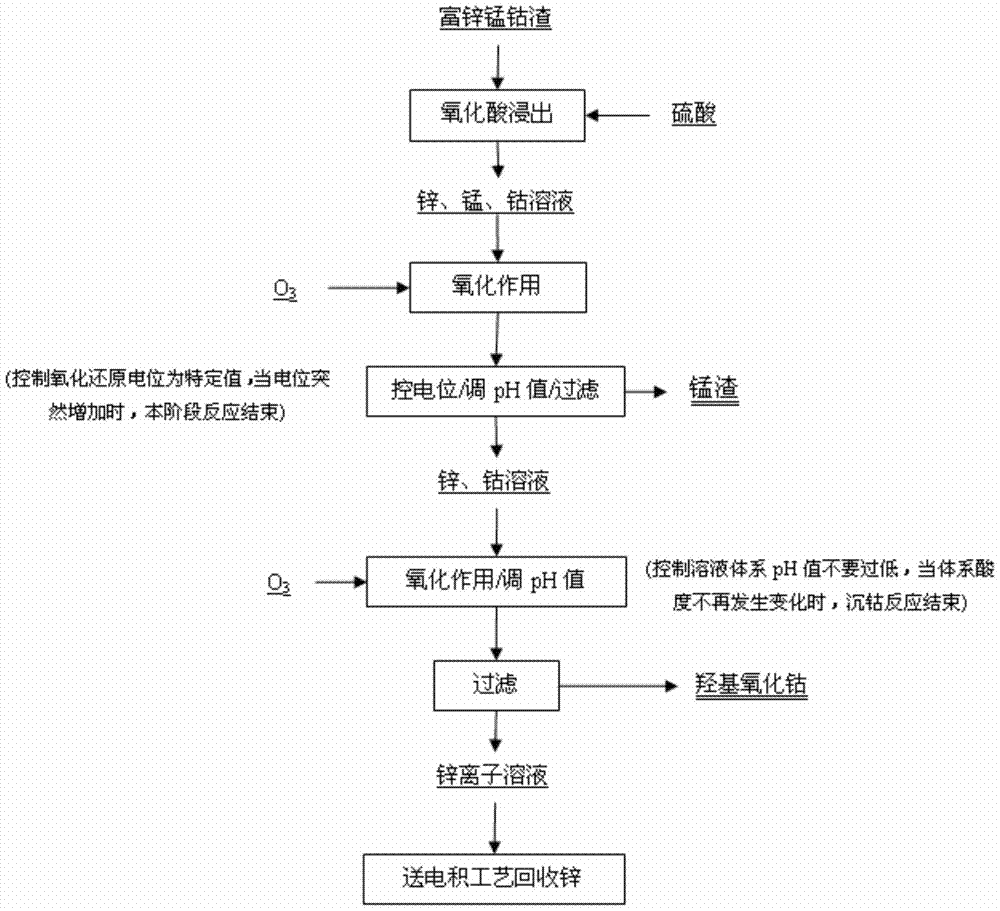
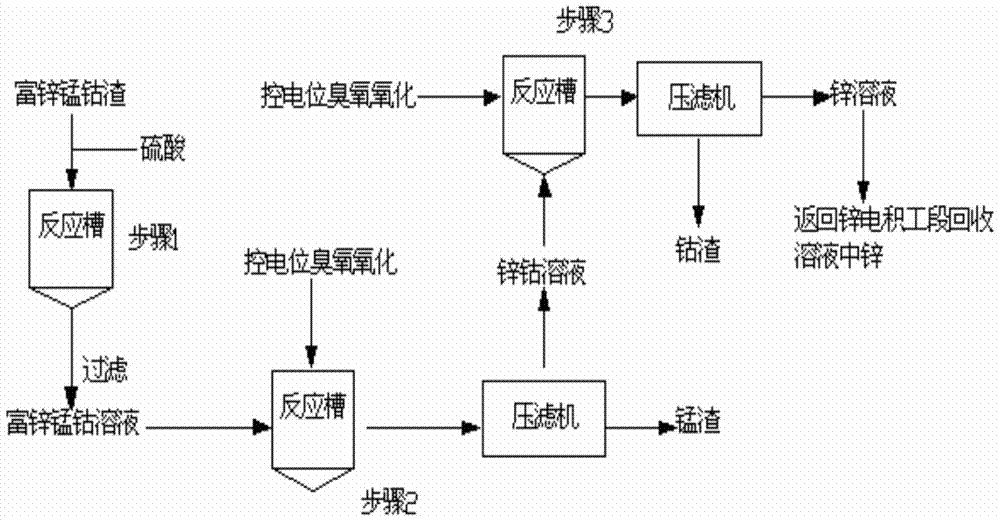
[0023] according to figure 1 Process, take 5kg zinc-rich manganese-cobalt slag material into the reaction tank, then add 0.2mol / LH 2 SO 4 The solution was 10 liters, the reaction temperature was controlled at 90° C., the stirring speed was 280 rpm, and the reaction time was 2 hours to obtain a solid-liquid mixture, which was centrifuged to obtain a polymetallic solution and a filter residue. This step is as figure 2 shown in step 1. The concentrations of zinc, manganese and cobalt in the multi-metal solution are 112g / L, 0.92g / L and 2.2g / L respectively.
[0024] Pass the multimetal solution obtained in step 1 into the reaction tank, and simultaneously pass ozone as an oxidizing agent for oxidation, manganese ions in the multimetal solution are preferentially oxidized and sink to the bottom, and are separated and recovered from the multimetal solution. During the reaction process, a potentiometer or an electrochemical workstation is used to detect the open circuit voltage d...
Embodiment 2
[0028] according to figure 1 Process, take 10kg zinc-rich manganese-cobalt slag material into the reaction tank, then add 0.2mol / LH 2 SO 4 The solution was 15 liters, the reaction temperature was controlled at 95° C., the stirring speed was 300 rpm, and the reaction time was 2 hours to obtain a solid-liquid mixture, which was centrifuged to obtain a polymetallic solution and a filter residue. This step is as figure 2 shown in step 1. The concentrations of zinc, manganese and cobalt in the multi-metal solution are 114g / L, 0.94g / L and 2.0g / L respectively.
[0029]Pass the multimetal solution obtained in step 1 into the reaction tank, and simultaneously pass ozone as an oxidizing agent for oxidation, manganese ions in the multimetal solution are preferentially oxidized and sink to the bottom, and are separated and recovered from the multimetal solution. During the reaction process, a potentiometer or an electrochemical workstation is used to detect the open circuit voltage d...
Embodiment 3
[0033] according to figure 1 Process, take 7kg zinc-rich manganese-cobalt slag material into the reaction tank, then add 0.2mol / LH 2 SO 4 The solution was 15 liters, the reaction temperature was controlled at 85° C., the stirring speed was 250 rpm, and the reaction time was 2.5 hours to obtain a solid-liquid mixture, which was centrifuged to obtain a polymetallic solution and a filter residue. This step is as figure 2 shown in step 1. The concentrations of zinc, manganese and cobalt in the multi-metal solution are 108g / L, 0.98g / L and 2.1g / L respectively.
[0034] Pass the multimetal solution obtained in step 1 into the reaction tank, and simultaneously pass ozone as an oxidizing agent for oxidation, manganese ions in the multimetal solution are preferentially oxidized and sink to the bottom, and are separated and recovered from the multimetal solution. During the reaction process, a potentiometer or an electrochemical workstation is used to detect the open circuit voltage...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com