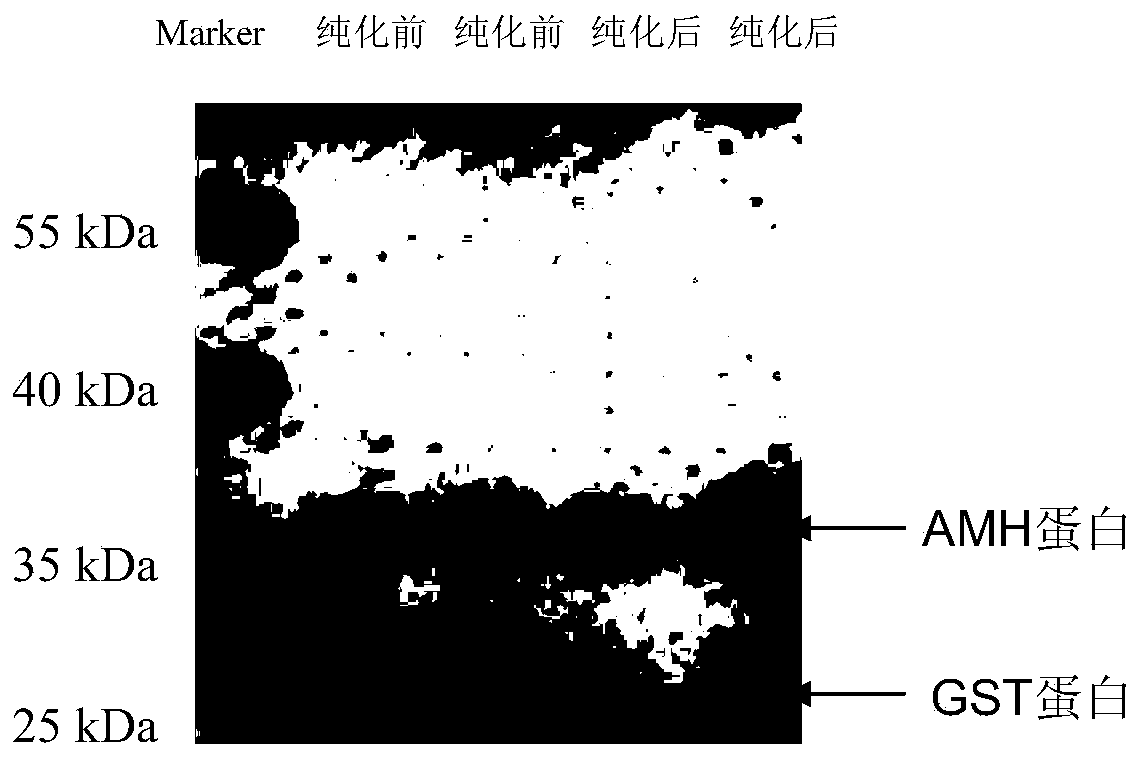
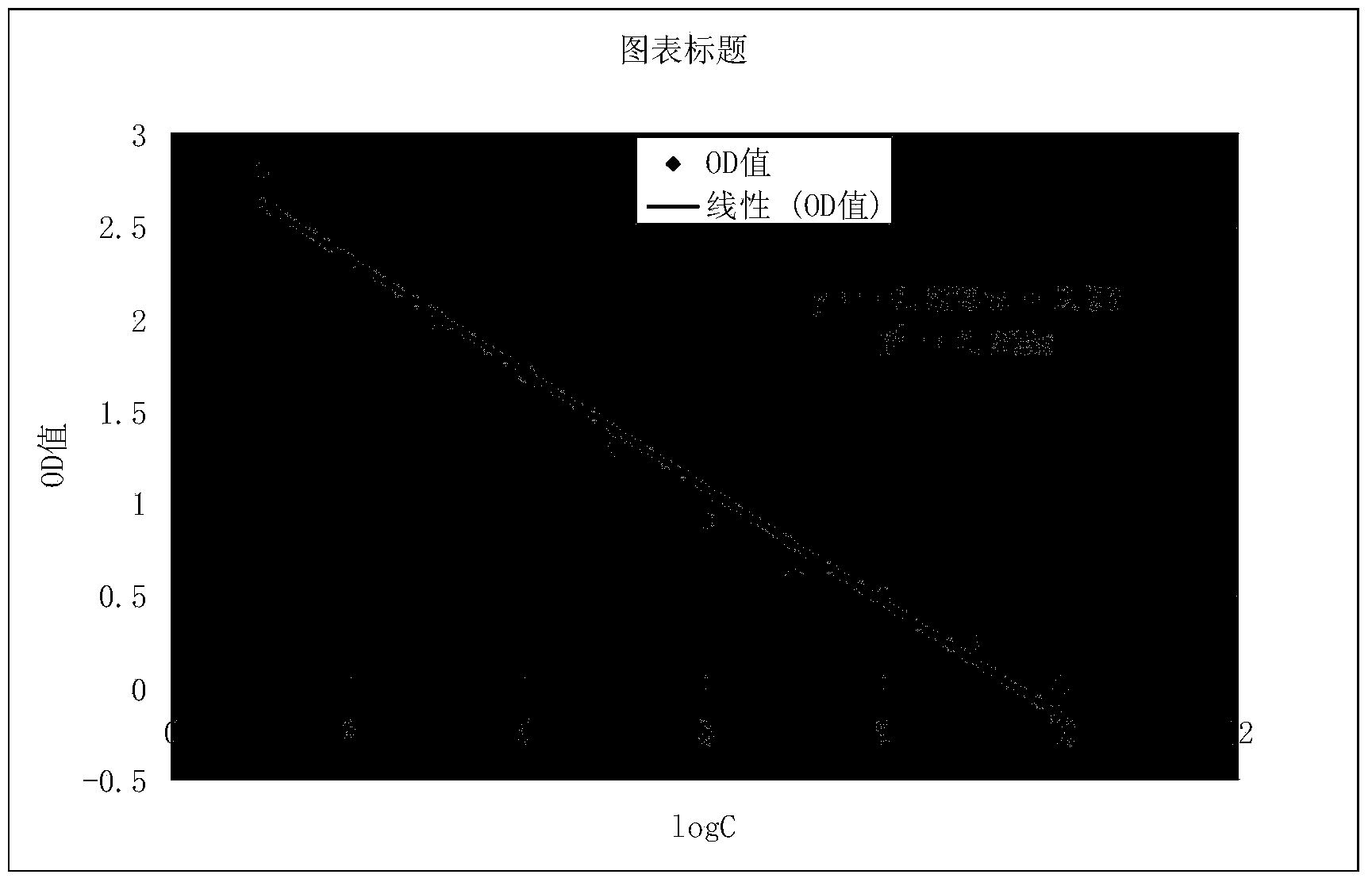
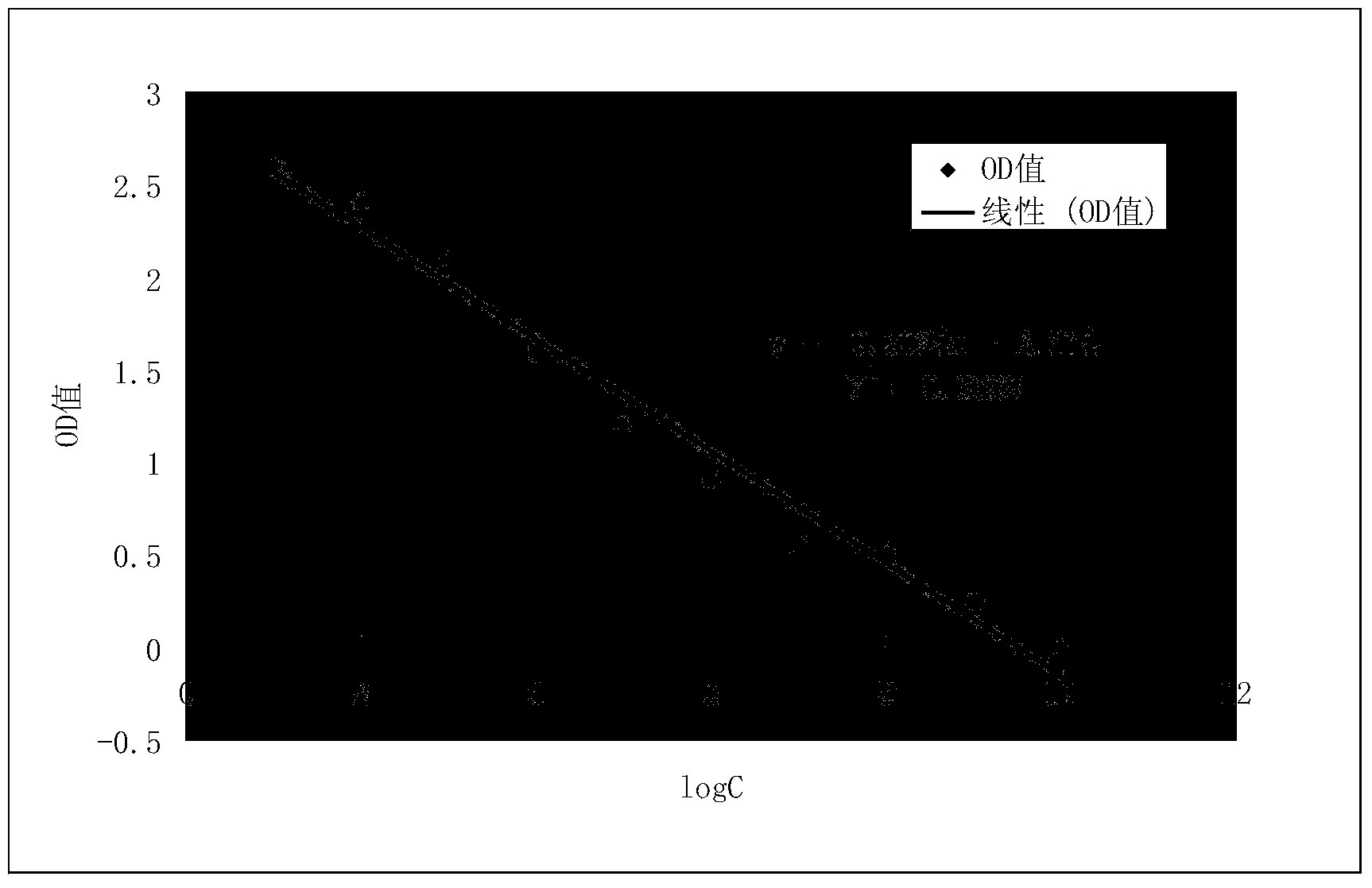
Preparation method and application of kit for double-sandwich immunofluorescence quantitative detection of human anti-Mullerian hormone (AMH) on basis of quantum dots
An immunofluorescence and quantitative detection technology, which is applied in the field of immunodiagnosis, can solve the problems of ineffective clinical promotion, many influencing factors, and not widely deployed, and achieve effective detection and risk assessment, improve resolution, and provide the effect of resolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0021] The present invention will be further described below in conjunction with specific examples, but not limited thereto.
[0022] 1) Construction of AMH prokaryotic expression vector
[0023] The immunogenic region of AMH is selected, its gene sequence is shown in SEQ ID NO.2, with a total of 297bp, and the corresponding amino acid sequence is shown in SEQ ID NO.1, with a total of 93 amino acids.
[0024] The specific primers designed to amplify the AMH immunogenic region sequence are as follows:
[0025] EcoR I Up primer: CCG GAATTC AGCGTAGACCTCCGCGCCGC (underlined part is the recognition sequence of endonuclease EcoR I) (SEQ ID NO.3),
[0026] Xho I Down primer: CCG CTCGAG CCGGCAGCCACACTCGGTGG (the underlined part is the recognition sequence of endonuclease Xho I) (SEQ ID NO.4).
[0027] Using chloroform to extract the total RNA of human ovarian cancer cells, amplify the AMH immunogenic region sequence through reverse transcription and PCR primers, digest the ampli...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com