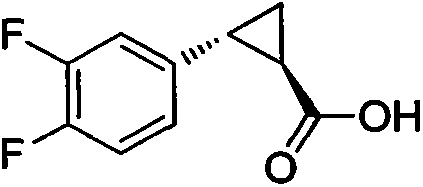
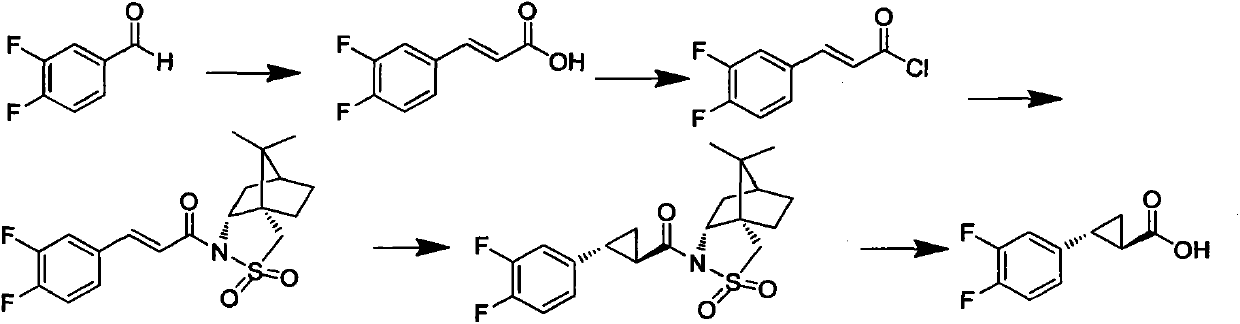
Method for preparing trans-(1R, 2R)-2-(3, 4-difluoro phenyl)-1-cyclopropane formic acid
A technology of cyclopropanecarboxylic acid and difluorophenyl, which is applied in the field of preparation of trans--2--1-cyclopropanecarboxylic acid, can solve the problems of rare raw materials, difficult separation, heavy pollution, etc., and achieves a simple preparation method, three-dimensional The effect of good selectivity and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
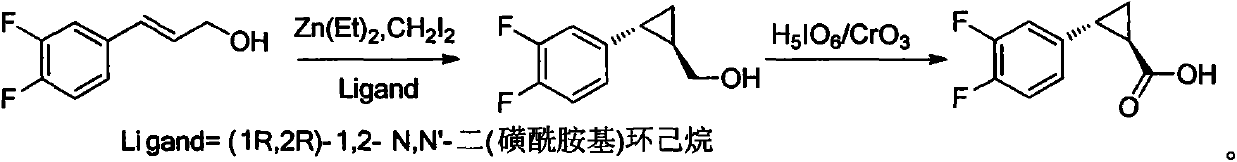
[0036] Example 1. Preparation of trans-(1R,2R)-2-(3,4-difluorophenyl)-1-cyclopropanemethanol:
[0037]
[0038] (E)-3-(3,4-difluorophenyl)prop-2-en-1-ol (1.70g, 10mmol) and (1R,2R)-1,2-N,N'-bis(3 , 5-dichlorobenzenesulfonamido)cyclohexane (5 mmol%) in 100 mL of anhydrous CH 2 Cl 2 solution, keep the temperature at -15-0°C, add Et 2 Zn (1mol / L n-hexane solution 20mL, 20mmol) and CH 2 I 2 (8.04g, 30mmol), keep the reaction for 5 hours, rise to room temperature, add 50mL of 2mol / L NaOH solution, and use 100mL CH 2 Cl 2After three extractions, the catalyst can be recovered by acidifying the aqueous layer. The organic layers were combined, washed once with saturated aqueous sodium chloride, dried over anhydrous Na2SO4, and the solvent was distilled off under reduced pressure to obtain trans-(1R,2R)-2-(3,4-difluorophenyl)-1- Cyclopropanemethanol 1.66g, yield 90%, ee value: 97% (HPLC, chiral column, 3% isopropanol n-hexane). IR (KBr): 3450, 362, 3012, 2927, 1865, 1605, 149...
Embodiment 2
[0039] Example 2. Preparation of trans-(1R,2R)-2-(3,4-difluorophenyl)-1-cyclopropanemethanol:
[0040] (E)-3-(3,4-difluorophenyl)prop-2-en-1-ol (1.70g, 10mmol) and (1R,2R)-1,2-N,N'-bis(3 , 5-dichlorobenzenesulfonamido)cyclohexane (5 mmol%) in 100 mL of anhydrous CH 2 Cl 2 solution, keep the temperature at -15-0°C, add Et 2 Zn (1mol / L n-hexane solution 21mL, 21mmol) and CH 2 I 2 (8.04g, 30mmol), heat preservation reaction for 5 hours, refer to Example 1 for post-treatment process, yield 94%, ee value: 98.9%.
Embodiment 3
[0041] Example 3, Preparation of trans-(1R, 2R)-2-(3,4-difluorophenyl)-1-cyclopropanemethanol
[0042] (E)-3-(3,4-difluorophenyl)prop-2-en-1-ol (1.70g, 10mmol) and (1R,2R)-1,2-N,N'-bis(3 , 5-dichlorobenzenesulfonamido)cyclohexane (10 mmol%) in 100 mL of anhydrous CH 2 Cl 2 solution, keep the temperature at -15-0°C, add Et 2 Zn (1mol / L n-hexane solution 25mL, 25mmol) and CH 2 I 2 (9.4g, 35mmol), heat preservation reaction for 5 hours, refer to Example 1 for post-treatment process, yield 98%, ee value: 99.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com