Tissue culture method of monocotyledons
A technique for monocotyledonous plants and seeds, which is applied in the field of tissue culture of monocotyledonous plants, can solve the problems of long screening period, high difficulty, and heavy workload, and achieve the effects of reducing the difficulty of selection, easy operation, saving time and cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
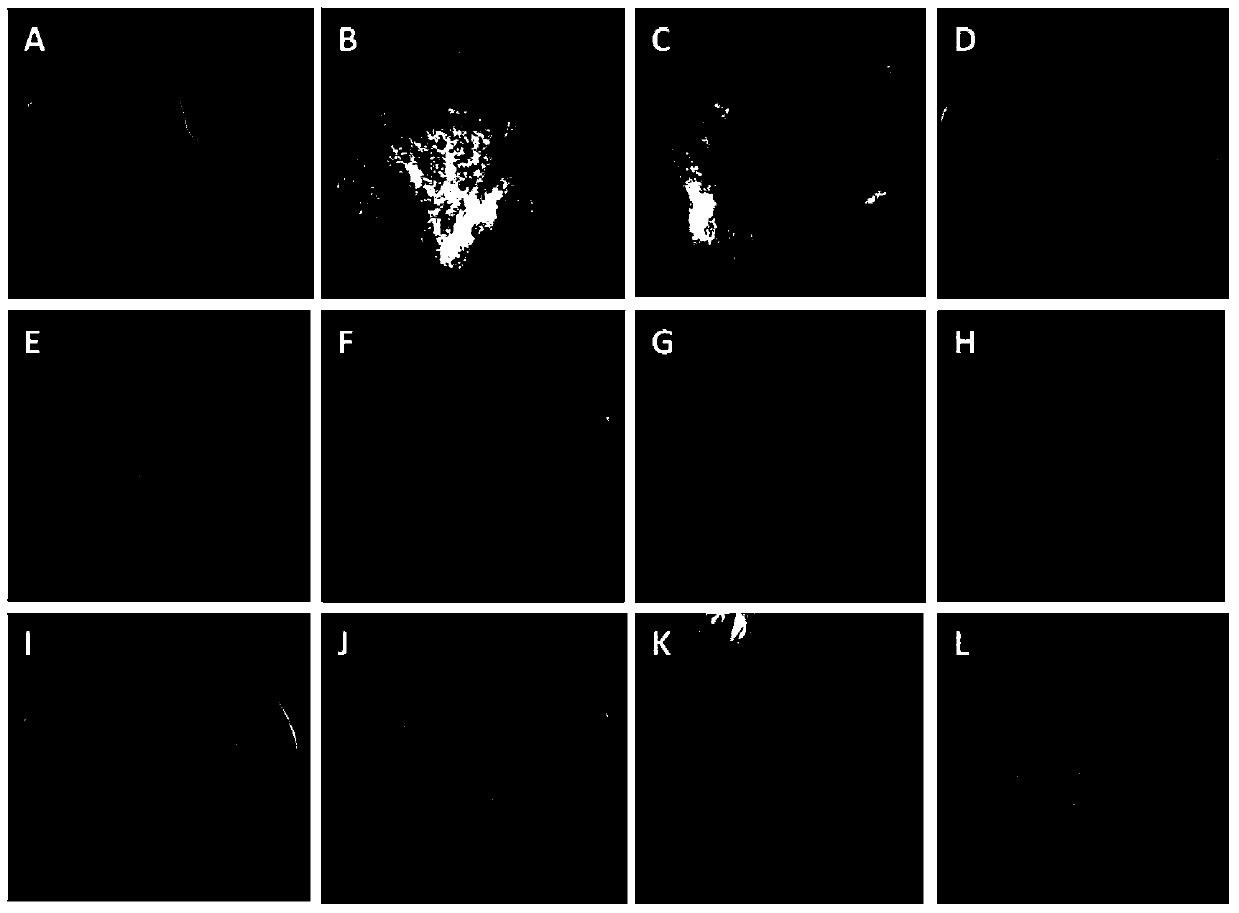
[0068] Embodiment 1, the acquisition and tissue culture of type II callus of switchgrass variety Alamo
[0069] Test material: mature seeds of switchgrass variety Alamo. Alamo is a lowland switchgrass with high biomass, flowering in autumn, moderate salt tolerance, suitable for most soil types, and is a common material for tissue culture of switchgrass. The tested Alamo mature seeds were produced in Texas, USA (25°50'N-36°30'N) in 2010.
[0070] (1) Seed disinfection
[0071] Select plump seeds of Alamo, sterilize with 5% (volume fraction) sodium hypochlorite aqueous solution for 1.5 h, wash with sterile deionized water 5 times, soak in sterile deionized water and place in an incubator at 4 °C for 3 days. After 3 days, it was treated with 5% (volume fraction) sodium hypochlorite aqueous solution for 30 minutes, and washed 5 times with sterile deionized water.
[0072] (2) Induction of callus
[0073] During callus induction, the following three media were selected as callu...
Embodiment 2
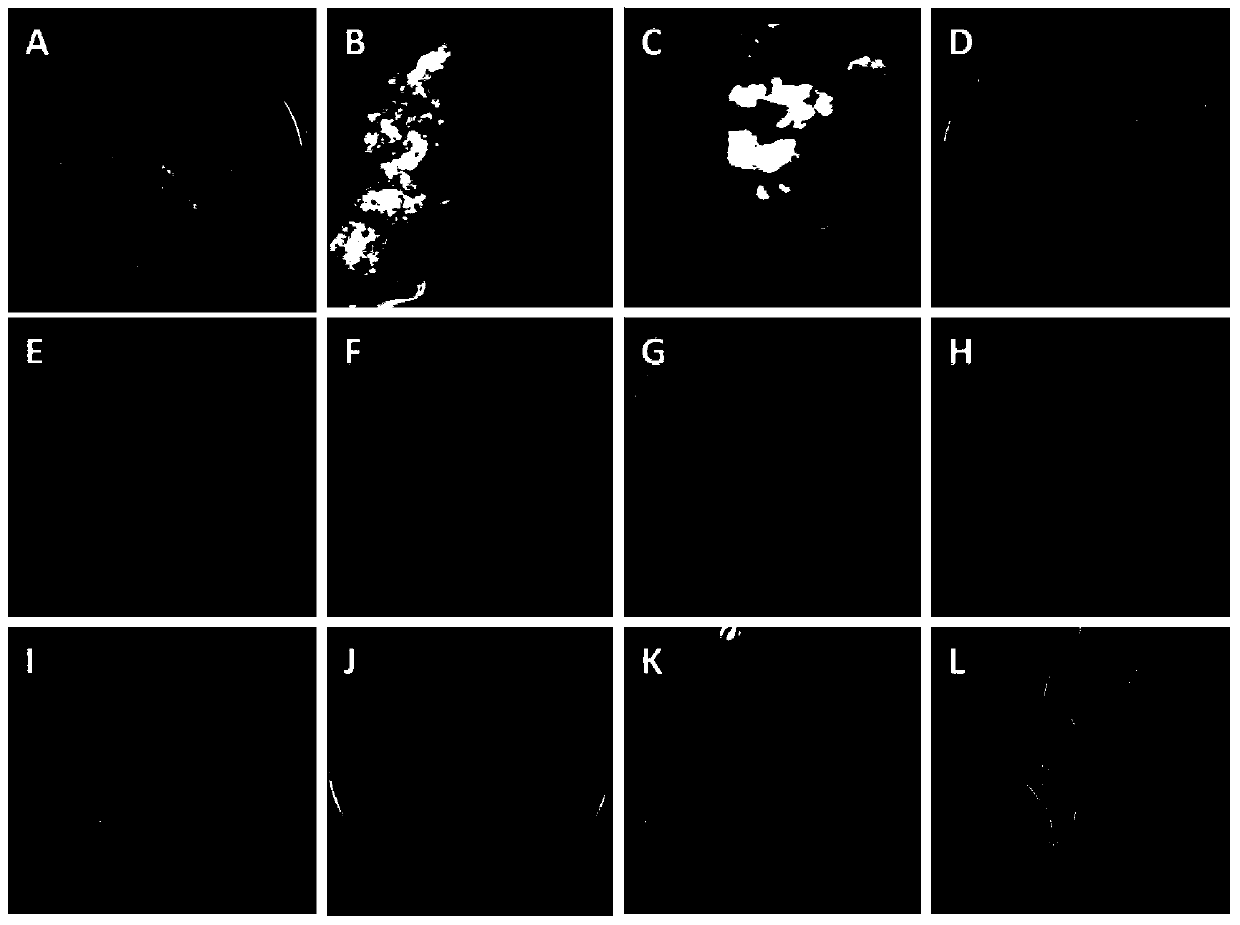
[0118] Example 2, the acquisition and tissue culture of type II callus of switchgrass variety Performer
[0119] Test material: mature seeds of switchgrass variety Performer. Performer is a lowland type switchgrass with high nutritional value, excellent forage quality and high biomass. It is a common material for switchgrass tissue culture. The mature seeds of Performer tested were produced in North Carolina, USA (33°50N-36°35'N) in 2010.
[0120] (1) Seed disinfection
[0121] Refer to step (1) of Example 1 for operation.
[0122] (2) Induction of callus
[0123] Refer to step (2) of Example 1 for operation.
[0124] The callus induction rate is shown in Table 5. For treatment ① (direct induction), the callus induction rate on MB solid medium was 53.34%, the callus induction rate on MS7 solid medium was 41.11%, and the callus induction rate on NB7 solid medium The callus induction rate on the medium was 52.78%, and there was no significant difference in the callus induc...
Embodiment 3
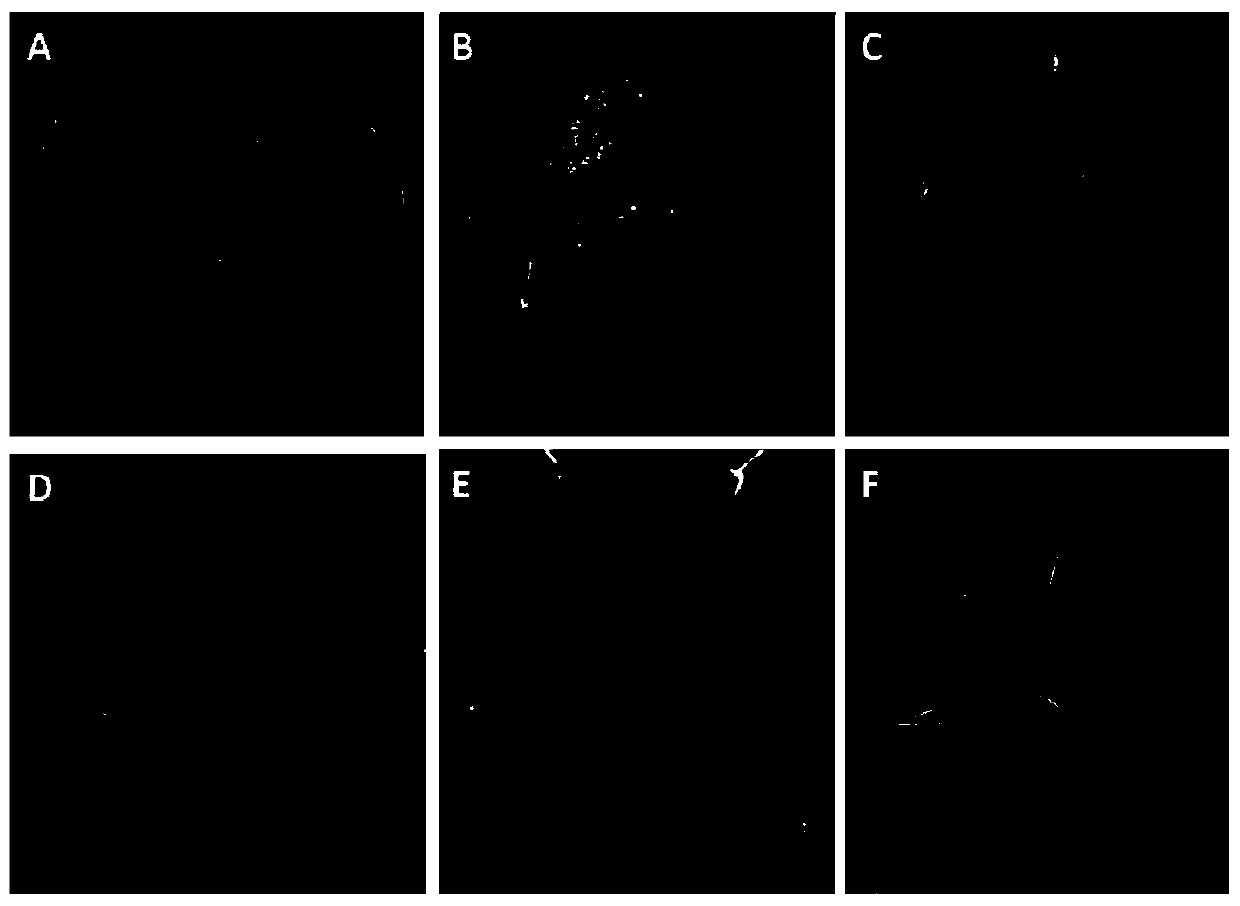
[0148] Example 3, the acquisition and tissue culture of type II callus of switchgrass variety Carthage
[0149] Test material: mature seeds of switchgrass variety Carthage. Carthage is a transitional switchgrass variety with many leaves, fast-growing stalks, and green in early spring. Its biomass exceeds that of Midwestern varieties in the United States and has high nutritional value. Due to difficult regeneration, it is rarely used in tissue culture. The mature seeds of Carthage tested were produced in North Carolina (33°50'N-36°35'N) in 2010.
[0150] (1) Seed disinfection
[0151] Refer to step (1) of Example 1 for operation.
[0152] (2) Induction of callus
[0153] Refer to step (2) of Example 1 for operation.
[0154] The callus induction rate is shown in Table 9. For treatment ① (direct induction), the callus induction rate on MB solid medium was 60.56%, the callus induction rate on MS7 solid medium was 61.67%, and the callus induction rate on NB7 solid medium The ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com