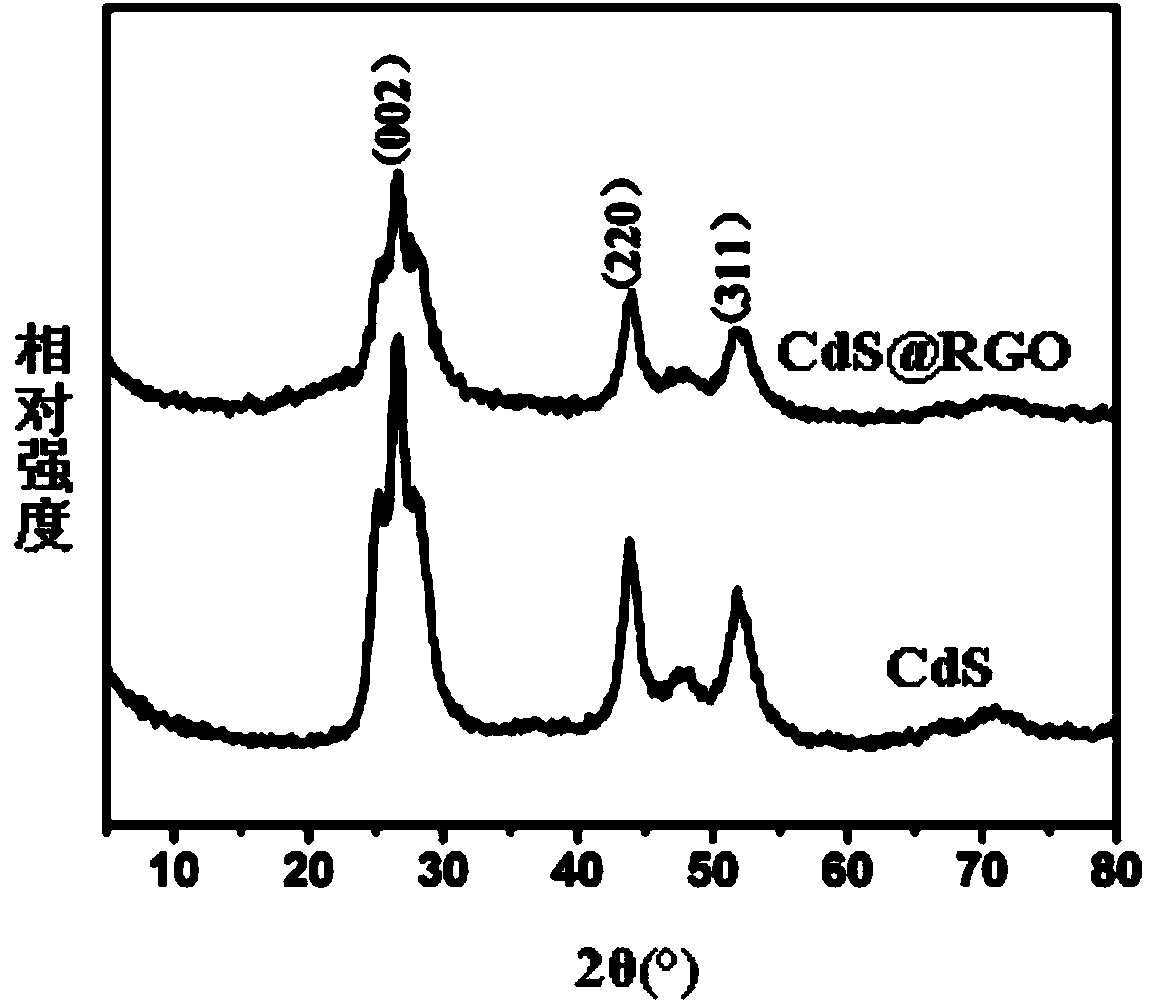
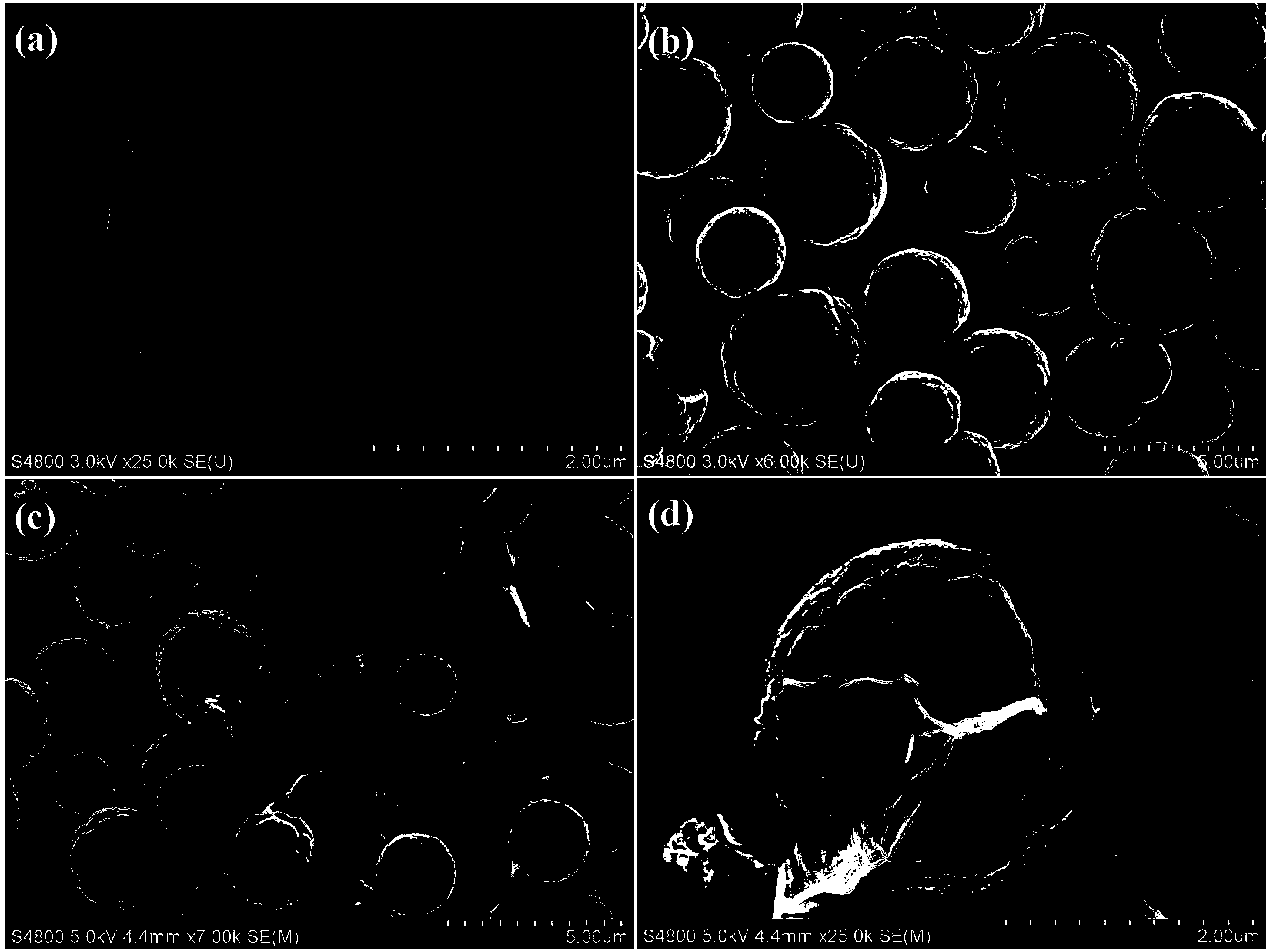
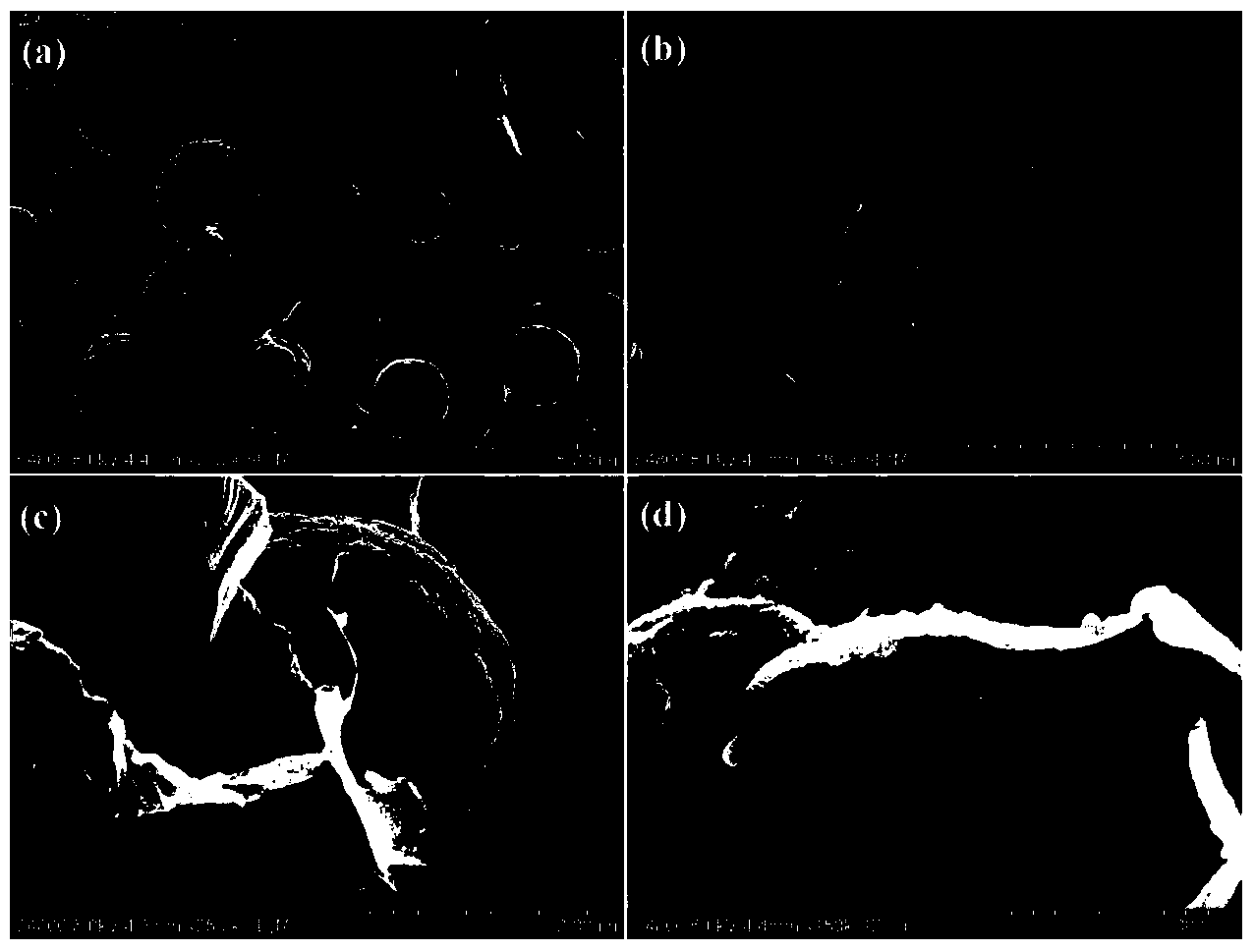
Method for preparing photocatalytic material with graphene-covered cadmium sulfide nuclear shell structure
A photocatalytic material, graphene-wrapped technology, applied in chemical instruments and methods, physical/chemical process catalysts, chemical/physical processes, etc., can solve problems such as reducing photocatalytic efficiency, limiting application, instability, etc., to achieve high photocatalysis The effect of reaction efficiency, uniform size and regular morphology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 0.006mol of cadmium chloride (CdCl 2 ) was dissolved in 40mL of distilled water, and 0.006mol of sodium thiosulfate (Na 2 S 2 o 3 ), continue to stir for 30min. Then this mixed solution is filled in the liner of 70mL polytetrafluoroethylene, and the filling amount is 60% of the volume of the liner of polytetrafluoroethylene. Put the bushing into a stainless steel kettle, and put the hydrothermal kettle into an oven for hydrothermal reaction at 120°C for 12 hours. After the reaction was completed, the reactor was taken out and cooled naturally. A bright yellow precipitate appeared in the bushing of the reactor. The yellow precipitate was filtered and washed three times with distilled water and absolute ethanol. The obtained product was dried in an oven at 80° C. for 3 hours to obtain cadmium sulfide microspheres. Take 0.1 g of pre-prepared graphene oxide and ultrasonically disperse it in 400 mL of deionized water to form a graphene oxide suspension, then add 1 g of ...
Embodiment 2
[0031] 0.006mol of cadmium chloride (CdCl 2 ) was dissolved in 40mL of distilled water, and 0.006mol of sodium thiosulfate (Na 2 S 2 o 3 ), continue to stir for 30min. Then this mixed solution is filled in the liner of 70mL polytetrafluoroethylene, and the filling amount is 60% of the volume of the liner of polytetrafluoroethylene. Put this bushing into a stainless steel kettle, and put the hydrothermal kettle into an oven for hydrothermal reaction at 160°C for 24 hours. After the reaction was completed, the reactor was taken out and cooled naturally. A bright yellow precipitate appeared in the bushing of the reactor. The yellow precipitate was filtered and washed three times with distilled water and absolute ethanol. The obtained product was dried in an oven at 80° C. for 3 hours to obtain cadmium sulfide microspheres. Take 0.25 g of pre-prepared graphene oxide and ultrasonically disperse it in 400 mL of deionized water to form a graphene oxide suspension, then add 1 g o...
Embodiment 3
[0033] 0.012mol of cadmium chloride (CdCl 2 ) was dissolved in 40mL of distilled water, and 0.012mol of sodium thiosulfate (Na 2 S 2 o 3 ), continue to stir for 30min. Then this mixed solution is filled in the liner of 70mL polytetrafluoroethylene, and the filling amount is 60% of the volume of the liner of polytetrafluoroethylene. Put this bushing into a stainless steel kettle, and put the hydrothermal kettle into an oven for hydrothermal reaction at 140°C for 12 hours. After the reaction was completed, the reactor was taken out and cooled naturally. A bright yellow precipitate appeared in the bushing of the reactor. The yellow precipitate was filtered and washed three times with distilled water and absolute ethanol. The obtained product was dried in an oven at 80° C. for 3 hours to obtain cadmium sulfide microspheres. Take 0.5 g of pre-prepared graphene oxide and ultrasonically disperse it in 400 mL of deionized water to form a graphene oxide suspension, then add 1 g of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com