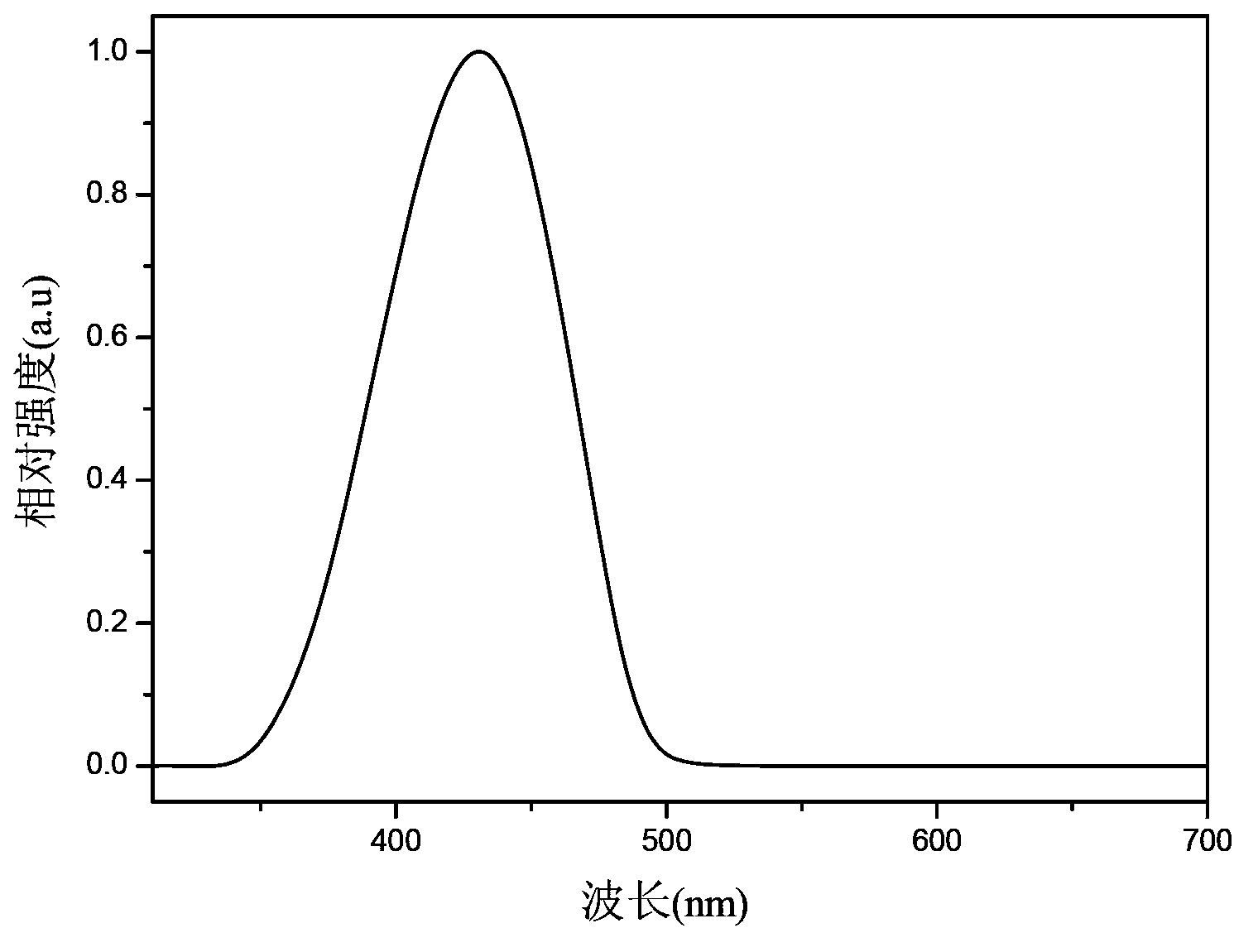
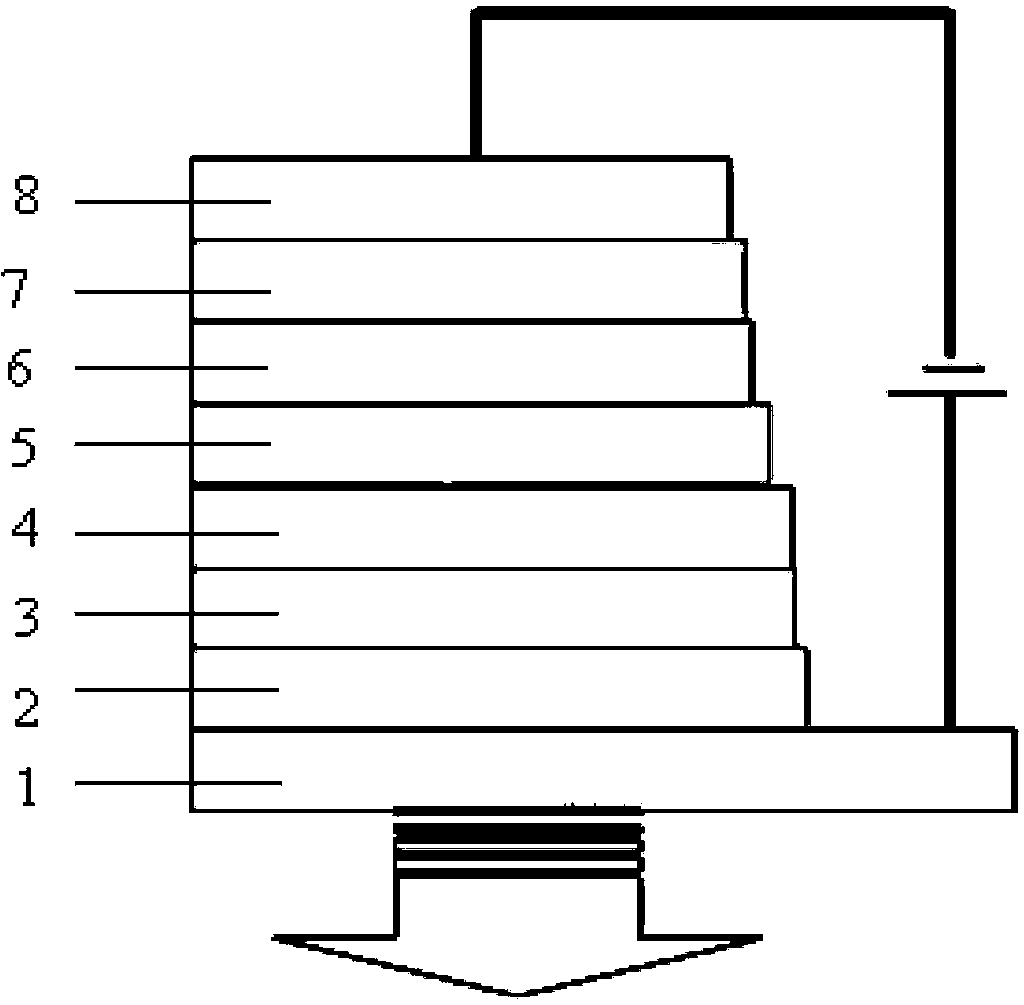
Organic semiconductor material containing carbazole, preparation method thereof, and organic electroluminescent device
An organic semiconductor and carbazole technology, which is applied in the field of organic electroluminescent devices, can solve the problems of lack of blue light materials and affect the life development of white light devices, etc., and achieve simple process, good hole transport ability and luminous efficiency, and simple preparation method Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
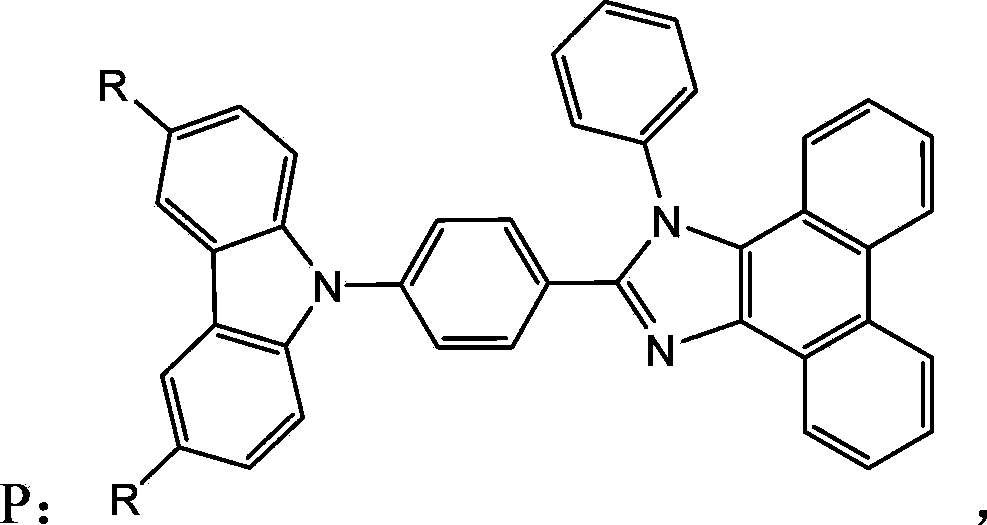
[0041] A carbazole-containing organic semiconductor material, namely 2-(4-(9H-carbazol-9-yl)phenyl)-1-phenyl-1H-phenanthrene[9,10-d]imidazole (named CzPPI), which is the compound P1 shown in the following structural formula:
[0042]
[0043] The preparation method of the above-mentioned carbazole-containing organic semiconductor material compound P1 comprises the following steps:
[0044] (1) Provide compound A (2-(4-bromophenyl)-1-phenyl-1H-phenanthrene[9,10-d]imidazole) and compound B1 (carbazole) represented by the following structural formula respectively,
[0045]
[0046] (2) Under nitrogen protection, add 2-(4-bromophenyl)-1-phenyl-1H-phenanthrene[9,10-d]imidazole 3mmol, carbazole 3.0mmol, toluene 25mL, and add Pd( OAc) 2 0.09mmol, t-BuONa 5.4mmol, (t-Bu) 3 PHBF 4 0.27mmol, reflux and stir at 110°C for coupling reaction for 20h. After the reaction, cool down, pour the reaction solution into a saturated ammonium chloride aqueous solution, extract three times...
Embodiment 2
[0050] A carbazole-containing organic semiconductor material, namely 2-(4-(3,6-methyl-9H-carbazol-9-yl)phenyl)-1-phenyl-1H-phenanthrene[9,10 -d] imidazole (named MCzPPI), which is the compound P2 shown in the following structural formula:
[0051]
[0052] The preparation method of the above-mentioned carbazole-containing organic semiconductor material compound P2 comprises the following steps:
[0053] (1) Compound A (2-(4-bromophenyl)-1-phenyl-1H-phenanthrene[9,10-d]imidazole) and compound B2 (3,6-dimethyl base carbazole),
[0054]
[0055] (2) Under nitrogen protection, add 2-(4-bromophenyl)-1-phenyl-1H-phenanthrene[9,10-d]imidazole 3mmol, 3,6-dimethylcarbazole 3.5mmol, di Add 0.03mmol of CuI to 25mL of toluene under stirring, add 0.09mmol of o-phenanthroline and CuI for co-catalysis, 18.0mmol of KOH, and reflux and stir the coupling reaction at 130°C for 40h. After the reaction, cool down, pour the reaction solution into dilute hydrochloric acid solution, extract ...
Embodiment 3
[0058] A carbazole-containing organic semiconductor material, namely 2-(4-(3,6-ethyl-9H-carbazol-9-yl)phenyl)-1-phenyl-1H-phenanthrene[9,10 -d] imidazole (named ECzPPI), which is the compound P3 shown in the following structural formula:
[0059]
[0060] The preparation method of the above-mentioned carbazole-containing organic semiconductor material (i.e. compound P3) comprises the following steps:
[0061] (1) Compound A (2-(4-bromophenyl)-1-phenyl-1H-phenanthrene[9,10-d]imidazole) and compound B3 (3,6-diethyl base carbazole),
[0062]
[0063] (2) Add 4.0mmol of 2-(4-bromophenyl)-1-phenyl-1H-phenanthrene[9,10-d]imidazole and 6.0mmol of 3,6-diethylcarbazole sequentially under argon protection , o-dichlorobenzene 25mL, add Cu powder 6mmol under stirring, add 1,4,7,10,13,16-hexaoxacyclooctadecane (18-crown-6) 1.0mmol as a phase transfer catalyst, K 2 CO 3 27.0 mmol was refluxed and stirred at 185° C. for 48 h for the coupling reaction. After the reaction, pass thr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com