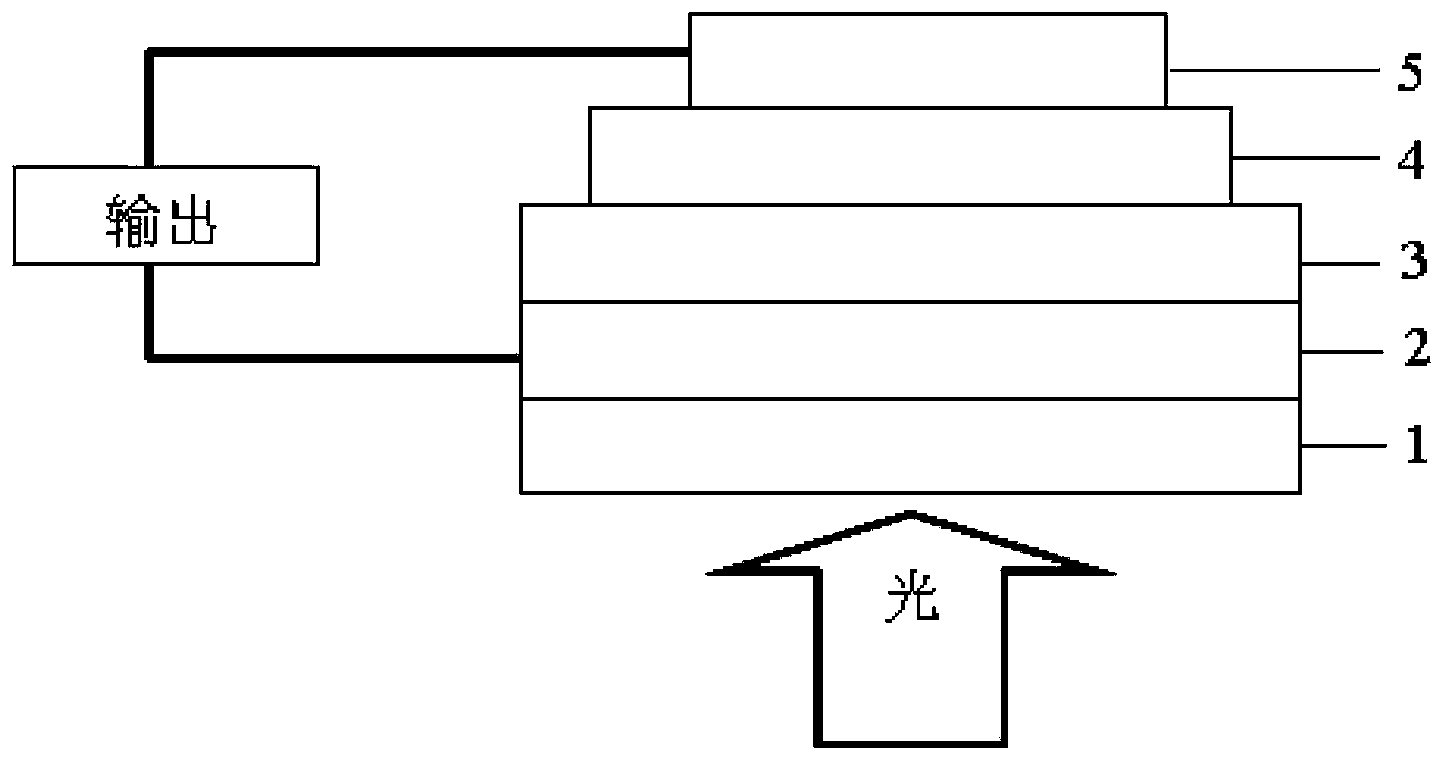
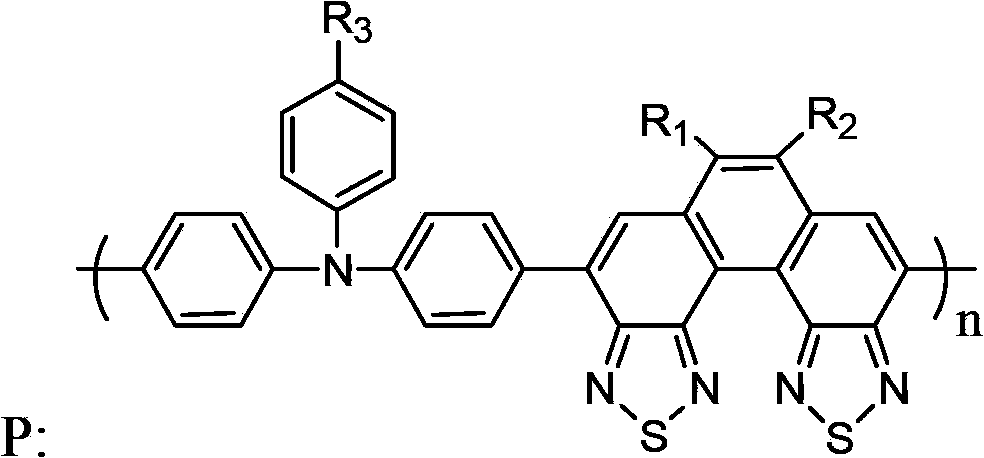
Triphenylamine-benzodi(benzothiadiazole) containing copolymer, preparation and application thereof
A technology of benzothiadiazole and triphenylamine, which is applied in the field of triphenylamine-benzodipolymer and its preparation and application, can solve the problems of low energy conversion efficiency, achieve reduced preparation costs, good electron transport properties, Effect of improving solubility and film-forming properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] A triphenylamine-benzobis(benzothiadiazole) copolymer, namely poly{triphenylamine-6,7-bis(3,7-dimethyl)octyl-benzo[2,1-e3 ,4-e] bis(benzothiadiazole)} (n=55), denoted as copolymer P1:
[0066]
[0067] The preparation method comprises the following steps:
[0068] 1. Preparation of 4,4'-bis((4,4,5,5-tetramethyl-1,3,2-dioxaborolane)triphenylamine (A1), including the following steps:
[0069] Provide compound C1, namely 4,4'-dibromotriphenylamine;
[0070] Under the protection of nitrogen, compound C1 (12.10g, 0.03mol) and 200mL tetrahydrofuran were added to the three-necked flask, and the n-hexane solution (25.2mL, 2.5M , 0.06mol), continue to stir the reaction for 2h, then add 2-isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (13mL, 0.06mol), Stir overnight at room temperature, add the reaction solution after the reaction to 60 mL of saturated aqueous sodium chloride solution to terminate the reaction, extract with anhydrous ether, dry over anhydrous sodium sul...
Embodiment 2
[0097] A triphenylamine-benzobis(benzothiadiazole) copolymer, poly{4”-decyltriphenylamine-6-(3,7-dimethyl)octyl-7-n-eicosyl -Benzo[2,1-e:3,4-e]bis(benzothiadiazole)} (n=80), denoted as copolymer P2:
[0098]
[0099] The preparation method comprises the following steps:
[0100] 1. The preparation method of 4"-decyl-4,4'-di((4,4,5,5-tetramethyl-1,3,2-dioxaborolane) triphenylamine (A2) includes The following steps:
[0101] Provide compound C2, namely 4"-decyl-4,4'-dibromotriphenylamine;
[0102] Under the protection of nitrogen, compound C2 (5.43g, 0.01mol) and 80mL tetrahydrofuran were added to the three-necked flask, and the n-hexane solution (8.4mL, 2.5M , 0.02mol), continue to stir the reaction for 2h, then add 2-isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (4.3mL, 0.02mol) , stirred at room temperature overnight, the reaction solution after the reaction was added into 60mL saturated aqueous sodium chloride solution to terminate the reaction, extracted with an...
Embodiment 3
[0118] A triphenylamine-benzobis(benzothiadiazole) copolymer, namely poly{4”-octyloxytriphenylamine-6,7-bis(3,7-dimethyl)octyl-benzo [2,1-e:3,4-e]bis(benzothiadiazole)} (n=65), denoted as copolymer P3:
[0119]
[0120] The preparation method comprises the following steps:
[0121] 1. Preparation method of 4"-octyloxy-4,4'-bis((4,4,5,5-tetramethyl-1,3,2-dioxaborolane)triphenylamine (A3) Include the following steps:
[0122] Provide compound C3, namely 4"-octyloxy-4,4'-dibromotriphenylamine;
[0123] Under the protection of nitrogen, compound C3 (5.31g, 0.01mol) and 80mL tetrahydrofuran were added to the three-necked flask, and the n-hexane solution (8.4mL, 2.5M , 0.02mol), continue to stir the reaction for 2h, then add 2-isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (4.3mL, 0.02mol) , stirred at room temperature overnight, the reaction solution after the reaction was added into 60mL saturated aqueous sodium chloride solution to terminate the reaction, extracted wit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com