Method for determining dissolution rate of rosuvastatin calcium preparation
A technology of rosuvastatin calcium and rosuvastatin, applied in the field of medicine, can solve the problems of inability to accurately reveal the dissolution rate, affecting the correctness of pharmaceutical preparations and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] Chromatographic conditions: AgiLent Extend-C18 (4.6*250mm, 5μm); acetonitrile as mobile phase A, and 0.02% trifluoroacetic acid aqueous solution as mobile phase B, 0~13min, B: 63%, 13~27min, B: 63 %~10%; Detection wavelength: 242nm; Flow rate: 1.0mL / min; Column temperature: 40℃; Injection volume: 10μL.
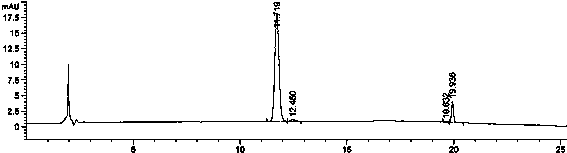
[0092] Dissolution curve establishment in pH1.0 hydrochloric acid solution: get rosuvastatin calcium tablet (three batches), parallel 6 parts, according to dissolution assay method (Chinese Pharmacopoeia 2010 edition one appendix XC second method), with 900ml pH1.0 Hydrochloric acid solution is used as the dissolution medium, the rotation speed is 50rpm, operate according to the law, take 4ml samples at 5min, 10min, 15min, 30min, 45min, 60min, and 90min for analysis, filter, carry out alkali neutralization treatment on the subsequent filtrate, and adjust the pH value to 7.0 as The test solution.
[0093] The result is as figure 1 , that is, the chromatogram of the dis...
Embodiment 2
[0110] Chromatographic conditions: AgiLent Extend-C18 (4.6*250mm, 5μm); acetonitrile as mobile phase A, and 0.02% trifluoroacetic acid aqueous solution as mobile phase B, 0~13min, B: 63%, 13~27min, B: 63 %~10%; Detection wavelength: 242nm; Flow rate: 1.0mL / min; Column temperature: 40℃; Injection volume: 10μL.
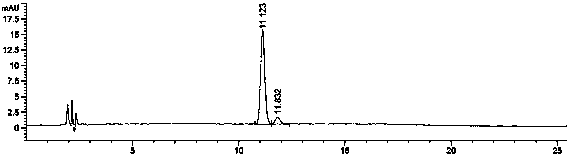
[0111] Establishment of dissolution curve in acetic acid-ammonium acetate buffer solution (pH4.5): Take rosuvastatin calcium tablets (three batches), 6 copies in parallel, according to the dissolution method (Appendix XC second method of Chinese Pharmacopoeia 2010 edition) , take acetic acid-ammonium acetate buffer (pH4.5) as the dissolution medium 900ml as the dissolution medium, the rotation speed is 50rpm, operate according to the law, take 4ml samples at 5min, 10min, 15min, 30min, 45min, 60min for analysis, filter, and take the subsequent filtrate As the test solution.
[0112] The result is as Figure 4 , Dissolution chromatogram of rosuvastatin calcium tablets i...
Embodiment 3
[0116] Chromatographic conditions: AgiLent Extend-C18 (4.6*250mm, 5μm); acetonitrile as mobile phase A, and 0.02% trifluoroacetic acid aqueous solution as mobile phase B, 0~13min, B: 63%, 13~27min, B: 63 %~10%; Detection wavelength: 242nm; Flow rate: 1.0mL / min; Column temperature: 40℃; Injection volume: 10μL.
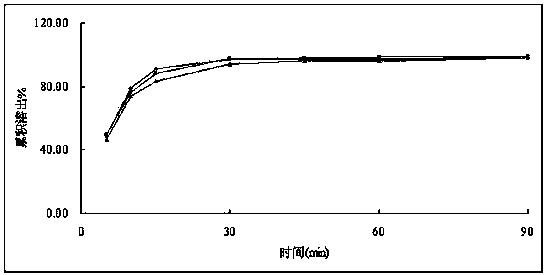
[0117] Dissolution curve establishment in 0.05M sodium citrate buffer solution (pH6.6): get rosuvastatin calcium tablet, parallel 6 parts, according to dissolution test method (Chinese Pharmacopoeia 2010 edition one appendix XC second method), with 900ml Phosphate buffer (pH 6.8) is used as the dissolution medium, and the rotating speed is 50rpm. Operate according to the law. Sampling 4ml at 5min, 10min, 15min, and 30min is analyzed, filtered, and the subsequent filtrate is taken as the test solution.
[0118] The result is as Figure 6 , that is, the dissolution chromatogram of rosuvastatin calcium tablets in 0.05M sodium citrate buffer (pH6.6).
[0119] Carry out di...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com