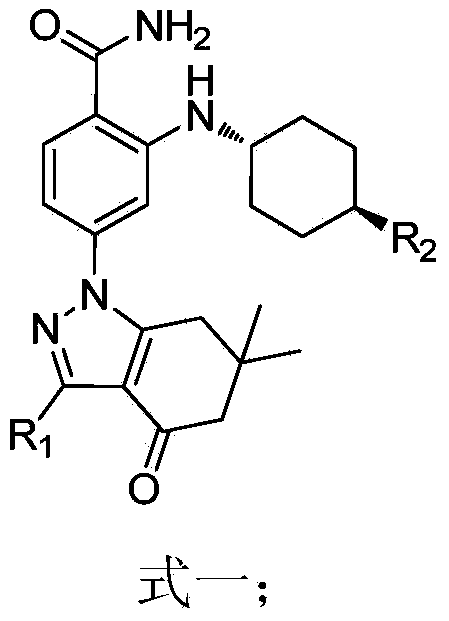
Application of Hsp90 inhibitor in preparing tumor multi-drug resistance reversal agents
A multi-drug resistance and inhibitor technology, applied in the field of biomedicine, can solve the problems of limited practical application value, toxic and side effects, and the targeting is easily degraded by DNA enzymes, so as to achieve good application prospects, enhance effect, improve effect of sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
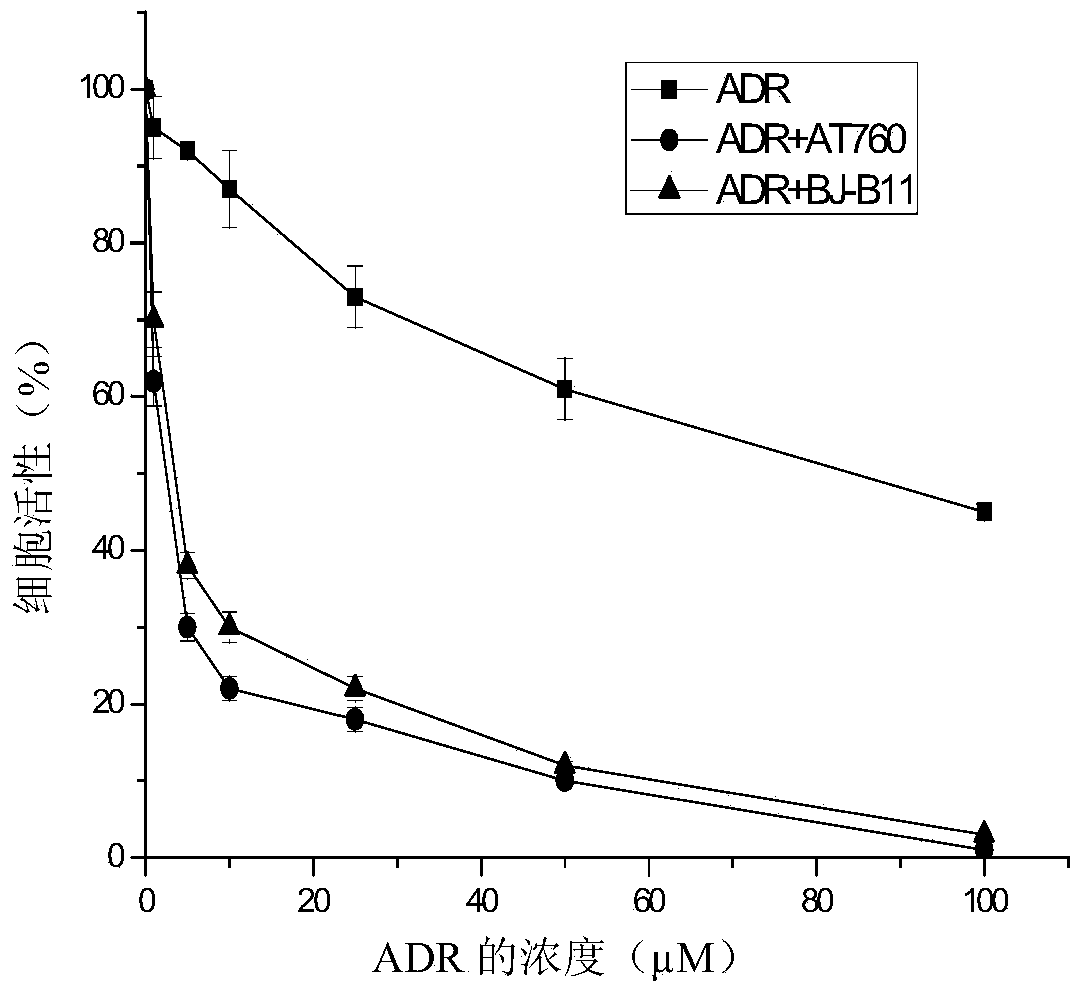
[0022] 2-(4-Hydroxycyclohexylamino)-4-(6,6-dimethyl-4-oxo-3-trifluoromethyl-4,5,6,7-tetrahydroindazole))benzamide (AT-760) reverse effect on multidrug resistant tumor cell K562 / ADR:
[0023] K562 / ADR cells were treated with 2×10 5 / mL density was inoculated in 96-well plates, and the cells were treated with different concentrations of compounds in groups. One group was added with 1 μM AT-760 and different concentrations (0, 1, 5, 10, 25, 50 and 100 , 25, 50 and 100 μM) of doxorubicin, placed at 37 ° C, 5% CO 2 Cultivate in the incubator for 48h. After the effect was completed, 20 μL of MTT (5 mg / mL) was added to each well, and the incubation was continued at 37° C. for 4 h, and then 90 μL of supernatant was carefully sucked out from each well. Subsequently, 100 μL of DMSO was added and incubated at 37° C. in the dark for 1 h. Measure the absorbance value of each well at a wavelength of 570nm on a BIO-RAD550 microplate reader (reference wavelength 630nm), calculate the cel...
Embodiment 2
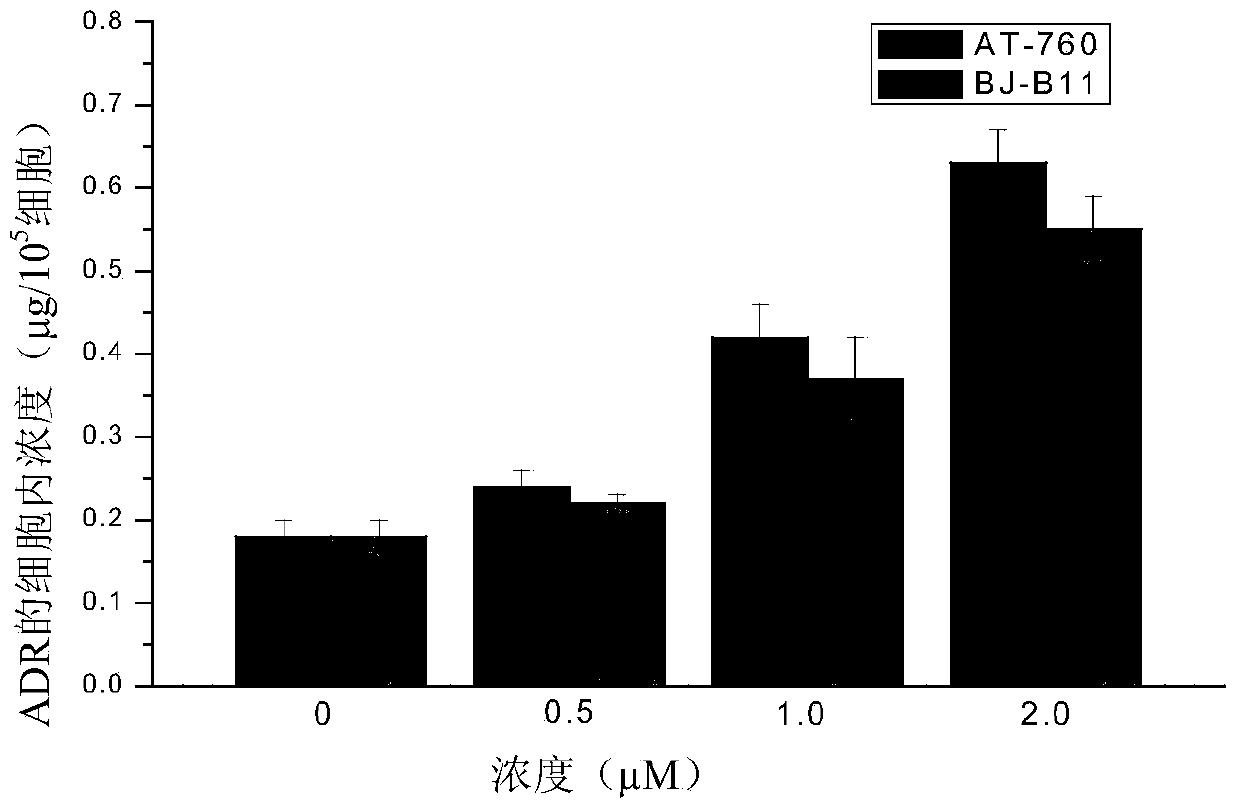
[0026] 2-(4-Hydroxycyclohexylamino)-4-(6,6-dimethyl-4-oxo-3-trifluoromethyl-4,5,6,7-tetrahydroindazole))benzamide Effect of (AT-760) on the accumulation of doxorubicin in multi-drug resistant tumor cells K562 / ADR:
[0027] K562 / ADR cells were treated with 2×10 5 / mL density was seeded in 96-well plates, and different compounds were added to treat the cells. The treatment method was to add 20 μM doxorubicin and different concentrations (0, 0.5, 1, 2 μM) of AT-760 to each group, and after culturing for 4 hours, wash the cells with cold PBS buffer solution (pH 7.4) 3 times to terminate the reaction. Collect the cells, add 0.3M HCl-50% ethanol solution to resuspend the cells, break the cells on an ultrasonic instrument and centrifuge for 15 minutes, take the supernatant and add hydrochloric acid-ethanol solution, and measure the fluorescence value of doxorubicin with a fluorescent microplate reader , the excitation and emission wavelengths are 470 nm and 580 nm, respectively.
...
Embodiment 3
[0030] 2-(4-acetoxycyclohexylamino)-4-(1-(3,6,6-trimethyl-4-oxo-4,5,6,7-tetrahydroindazole))benzamide ( Reversal effect of BJ-B11) on multidrug resistant tumor cell K562 / ADR:
[0031] K562 / ADR cells were treated with 2×10 5 / mL density was inoculated in 96-well plates, and the cells were treated with different concentrations of compounds in groups. One group was added with 1 μM BJ-B11 and different concentrations (0, 1, 5, 10, 25, 50 and 100 μM) of the chemotherapeutic drug doxorubicin (ADR), and the other group was only added with different concentrations (0, 1, 5, 10 , 25, 50 and 100 μM) of doxorubicin, placed at 37 ° C, 5% CO 2 Cultivate in the incubator for 48h. After the effect was completed, 20 μL of MTT (5 mg / mL) was added to each well, and the incubation was continued at 37° C. for 4 h, after which 90 μL of supernatant was carefully aspirated from each well. Subsequently, 100 μL of DMSO was added and incubated at 37° C. in the dark for 1 h. Measure the absorbance ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com