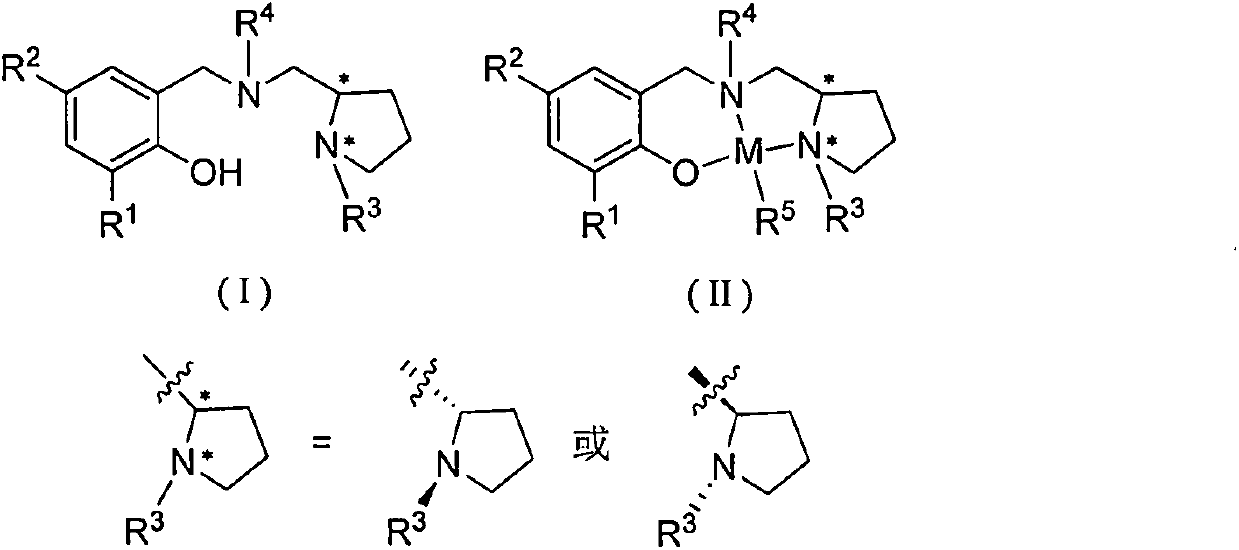
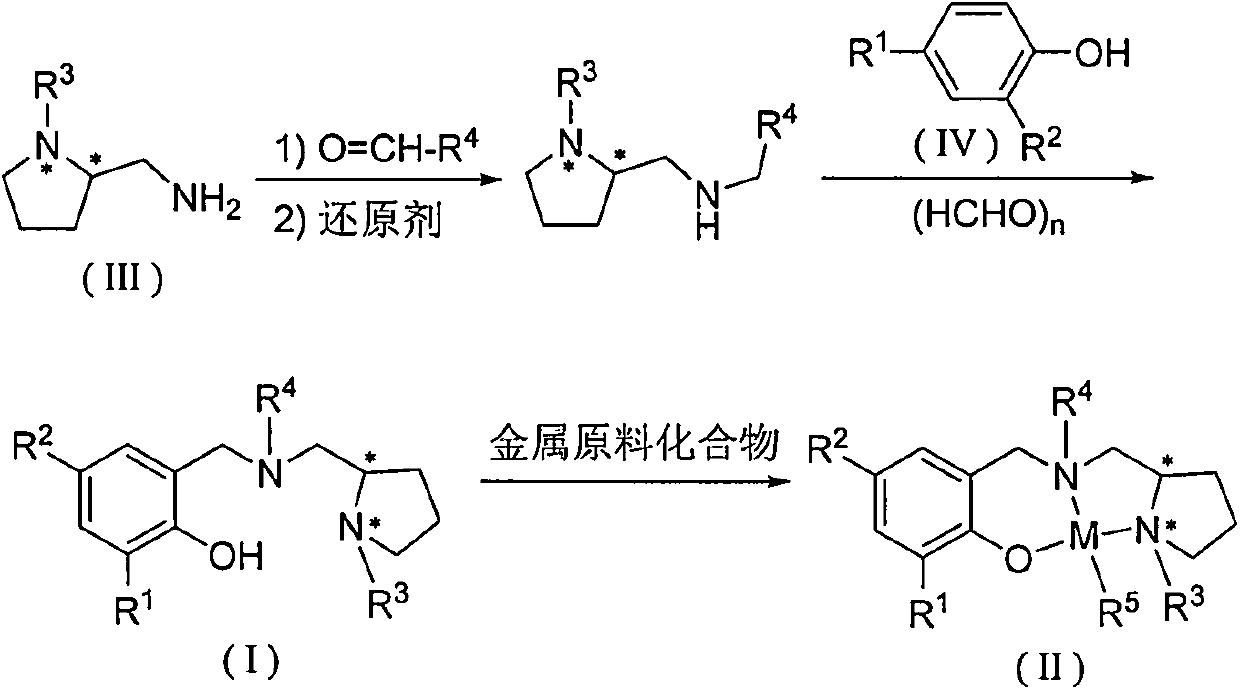
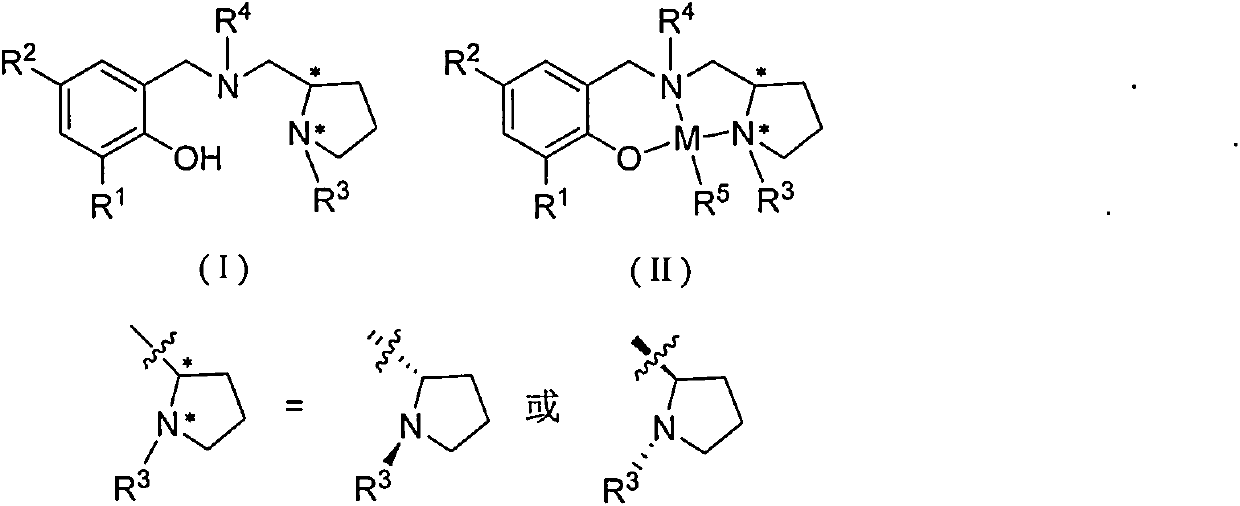
Chiral amino phenoxyl zinc and magnesium compound, and preparation method and application thereof
A technology of aminophenol oxyzinc and magnesium compounds, applied in the field of magnesium compounds and chiral aminophenoloxyzinc, which can solve the problems of high heterotactic selectivity and low selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Synthesis of Ligand L1
[0047] Add 1.22 g of benzaldehyde, 30 mL of absolute ethanol, and 1.28 g of (S)-1-ethyl-2-aminomethyltetrahydropyrrole into a 100 mL eggplant-shaped bottle, and heat to reflux for 24 hours. Add 0.76 g of sodium borohydride, stir for 3 hours, pour into water, extract the organic phase with dichloromethane, dry over anhydrous magnesium sulfate, and remove the solvent to obtain a light yellow viscous liquid. Add 30 mL of absolute ethanol, 0.6 g of paraformaldehyde, and 1.63 g of 2,4-dichlorophenol, and heat to reflux for 12 hours. The crude product was separated by column chromatography on silica gel to obtain red liquid L1 (2.32 g, 59.0%).
[0048]
[0049] 1 H NMR (400MHz, CDCl 3 ): δ7.22(m, 6H), 6.83(d, 4 J=2.4Hz), 3.84(d, 2 J=14.0Hz, 1H), 3.65(d, 2 J=13.0Hz, 1H), 3.53(d, 2 J=14.0Hz, 1H), 3.39(d, 2 J=13.0Hz, 1H), 3.06(m, 1H), 2.80-2.70(m, 1H), 2.60-2.50(m, 2H), 2.60-2.50(m, 1H), 2.21-2.08(m, 2H) , 2.01-1.94(m, 1H), 1.71-1.63(m, 2H), ...
Embodiment 2
[0051] Synthesis of Ligand L2
[0052] Add 1.22 g of benzaldehyde, 30 mL of absolute ethanol, and 1.28 g of (S)-1-ethyl-2-aminomethyltetrahydropyrrole into a 100 mL eggplant-shaped bottle, and heat to reflux for 24 hours. Add 0.76 g of sodium borohydride, stir for 3 hours, pour into water, extract the organic phase with dichloromethane, dry over anhydrous magnesium sulfate, and remove the solvent to obtain a light yellow viscous liquid. Add 30 mL of absolute ethanol, 0.6 g of paraformaldehyde, and 2.06 g of 2,4-di-tert-butylphenol, and heat to reflux for 12 hours. The crude product was separated by column chromatography on silica gel to obtain a colorless transparent liquid L2 (1.95 g, 44.7%).
[0053]
[0054] 1 H NMR (400MHz, CDCl 3 ): δ10.55(s, 1H), 7.35-7.23(m, 5H), 7.18(s, 1H), 6.85(s, 1H), 3.97(d, J=13.4Hz, 1H), 3.75(d, J=13.0Hz, 1H), 3.51(d, 2 J=13.4Hz, 1H), 3.37(d, 2J=13.0Hz, 1H), 3.09-3.02(m, 1H), 2.71-2.63(m, 1H), 2.50-2.38(m, 3H), 2.11-2.01(m, 1H), 2.03-1.8...
Embodiment 3
[0056] Synthesis of Ligand L3
[0057] Add 1.22 g of benzaldehyde, 30 mL of absolute ethanol, and 1.28 g of (S)-1-ethyl-2-aminomethyltetrahydropyrrole into a 100 mL eggplant-shaped bottle, and heat to reflux for 24 hours. Add 0.76 g of sodium borohydride, stir for 3 hours, cool to room temperature, pour into water, extract the organic phase with dichloromethane, dry over anhydrous magnesium sulfate, and remove the solvent to obtain a light yellow viscous liquid. Add 30 mL of absolute ethanol, 0.6 g of paraformaldehyde, and 3.30 g of 2,4-dicumylphenol, and heat to reflux for 12 hours. The crude product was separated by column chromatography on silica gel to obtain light brown liquid L3 (3.41 g, 60.9%).
[0058]
[0059] 1 H NMR (400MHz, CDCl 3 ): δ7.16(d, J=12.6Hz, 17H), 6.66(d, J=1.8Hz, 1H), 3.80(d, 2 J=13.6Hz, 1H), 3.46(d, 2 J=13.8Hz, 1H), 3.33(d, 2 J=13.6Hz, 1H), 3.13(d, 2 J=12.8Hz), 2.97-2.86(m, 1H), 2.55(dq, J=14.7, 7.3Hz, 1H), 2.28(dt, J=15.7, 7.9Hz, 3H), 2.01-1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com