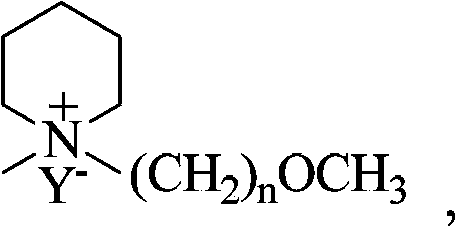Piperidines ionic liquid, and preparation method and application thereof
An ionic liquid, piperidine technology, applied in the preparation of sulfonates, sulfonic acid amides, organic chemistry, etc., can solve the problems of explosion, gas leakage, unsafe use, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
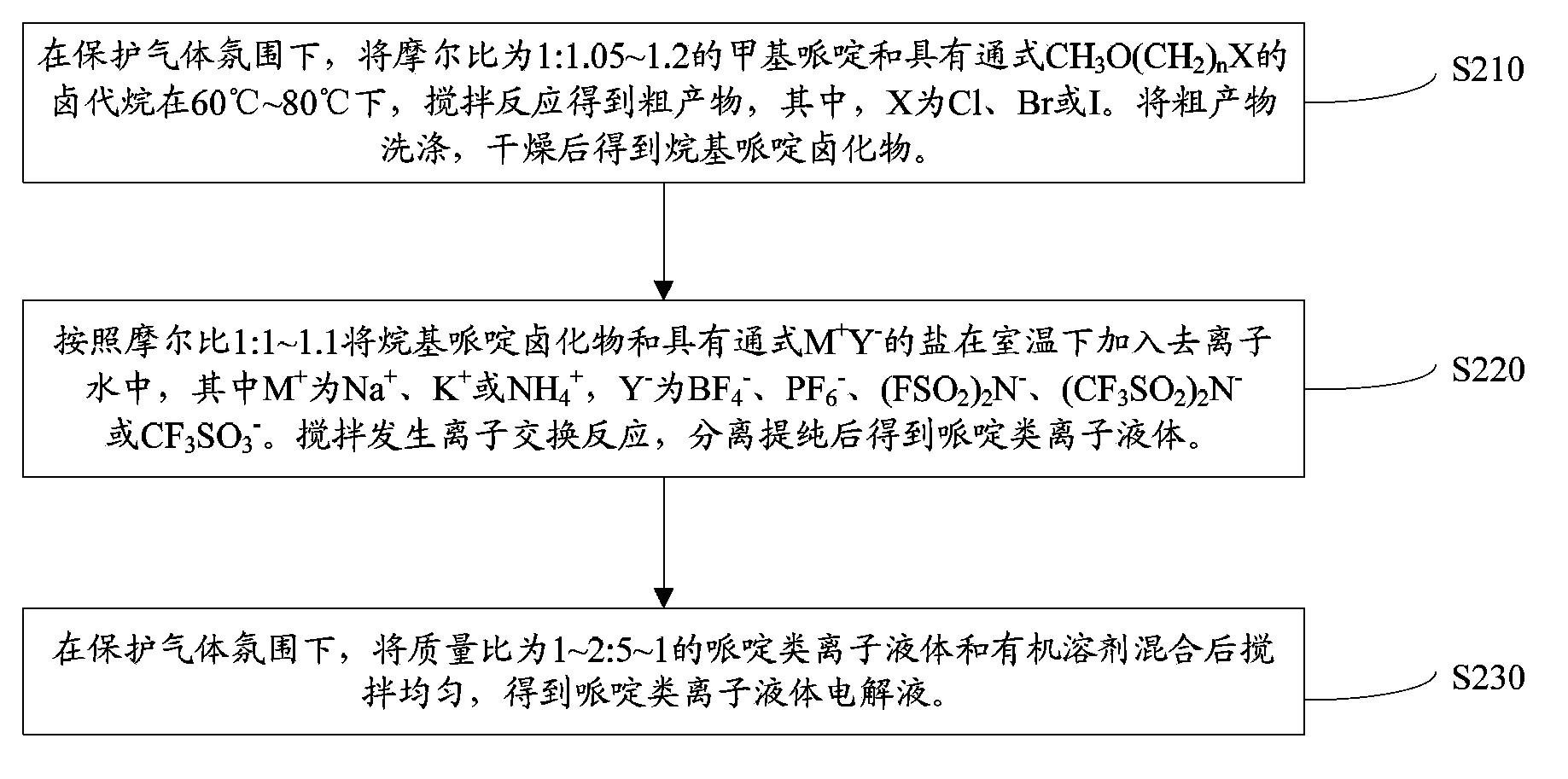
[0041] Such as figure 1 The preparation method of the above-mentioned piperidine class ionic liquid shown, comprises the following steps:
[0042] S110. Under a protective gas atmosphere, methylpiperidine having a molar ratio of 1:1.05 to 1.2 and a general formula CH 3 O(CH 2 ) n The alkyl halide of X is stirred and reacted at 60°C to 80°C to obtain a crude product, wherein X is Cl, Br or I. The crude product is washed and dried to give the alkylpiperidine halide.
[0043] Alkyl piperidine halides have the general structural formula:
[0044]
[0045] Wherein, n is 2, 3 or 4, X - for Cl - 、Br - or I - .
[0046] Methylpiperidine and have the general formula CH 3 O(CH 2 ) n The alkyl halide of X will undergo a nucleophilic substitution reaction, in which methylpiperidine is the nucleophile. During the reaction, the methyl piperidine will replace the CH with the general formula CH 3 O(CH 2 ) n The halogen in the haloalkane of X, the halogen leaves have the gen...
Embodiment 1
[0115] (1) Preparation of piperidine-based ionic liquids.
[0116] Into a 250mL flask were added 1mol of methylpiperidine and 1.1mol of methoxychloroethane. in N 2 Under the protection of the atmosphere, the temperature was raised to 70° C., and the reaction was stirred for 40 h. After standing to cool, the reaction product was washed three times with ethyl acetate, and then dried in vacuo at 80° C. to obtain light yellow solid N-methoxyethyl-N-methylpiperidinium chloride salt with a yield of 80 %.
[0117] Add 0.5mol of N-methoxyethyl-N-methylpiperidinium chloride salt, 0.53mol of NaBF to a 250mL flask 4 And 130mL deionized water, stirred at room temperature for 8h. The reacted solution was extracted three times with dichloromethane, the amount of dichloromethane used each time was 250 mL, and the organic phases of dichloromethane were combined. Then the dichloromethane organic phase was back-extracted several times with deionized water, the amount of deionized water eac...
Embodiment 2
[0121] (1) Preparation of piperidine-based ionic liquids.
[0122] Into a 250mL flask were added 1mol of methylpiperidine and 1.05mol of methoxybromopropane. Under the protection of Ar atmosphere, the temperature was raised to 60° C., and the reaction was stirred for 60 h. After standing to cool, the reaction product was washed three times with ethyl acetate, and then dried in vacuo at 80° C. to obtain light yellow solid N-methoxypropyl-N-methylpiperidinium bromide salt with a yield of 81 %.
[0123] Add 0.5 mol of N-methoxypropyl-N-methylpiperidinium bromide, 0.5 mol of KPF to the 250 mL flask 6 and 100 mL of deionized water, stirred at room temperature for 20 h, extracted the reacted solution three times with dichloromethane, each time the amount of dichloromethane was 250 mL, and combined the organic phases of dichloromethane. Then the dichloromethane organic phase was back-extracted several times with deionized water, the amount of deionized water each time was 60mL, un...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com